Gemstones: Malachite
|





 |
Malachite — a talisman for children.
Color: Malachite is an opaque, banded stone, the colors in the bands range from a very light green to almost deep green.
Description: Cu2CO3(OH)2 , Malachite is a semi-precious stone and also a valuable copper ore, hydrous copper carbonate. It is responsible for the green color of tarnished copper and bronze. Because of its distinctive bright green color and its presence in the weathered zone of nearly all copper deposits, malachite serves as a prospecting guide for that metal. Malachite has been used as an ornamental stone and as a gemstone.
The name’s origin: Malachite derives its name from Greek word malakos – soft. According to another theory the word malachite comes from Greek malhe, which means grass.
Wedding anniversary: Malachite is the anniversary gemstone for the 13th year of marriage.
Care and treatment: Malachite is especially fragile. Protect malachite from scratches and sharp blows. Also avoid large temperature changes. Do not clean malachite in a home ultrasonic cleaner. Washing malachite in water will remove its protective polish. If setting or repairing in jewelry be careful of heat since a jeweler’s torch can damage the stone.
From the stone history: Mining Malachite began as early as 4000 BC by ancient Egyptians. In the Middle ages, malachite was worn to protect from black magic and sorcery. In Ancient Greece amulets for children were made of malachite.
In the New Stone Age came the discovery of the possibility of extracting certain metals from the ores in which they generally occur. Probably the first such material to be used was malachite, then already in use as a cosmetic and easily reduced to copper in a strong fire. It is impossible to be precise about the time and place of this discovery, but its consequences were tremendous. Namely it led to the search for other metallic ores, to the development of metallurgy.
Shopping guide: Malachite is a beautiful green earth stone with irregular black banding. It is easily recognized by its color, green streak, and silky or velvety lustre. It is beautiful in earrings, necklaces, and pendants. Imitation malachite has very regular black or white banding.
Healing ability: Malachite is said to aid in the regeneration of body cells, creates calm and peace, and aids one’s sleep.
Mystical power: A gorgeous stone, Malachite is worn by many to detect impending danger. This beautiful green stone offers bands of varying hues and is believed by many to lend extra energy. It is believed that gazing at Malachite or holding it relaxes the nervous system and calms stormy emotions. Malachite is said to bring harmony into one’s life. It is also believed that malachite gives knowledge and patience. Malachite is used as a children’s talisman to ward off danger and illness. It is attached to infant’s cradles.
Deposits: The most important mine is in Zaire. Notable occurrences are in Ural, Siberia, France, South Australia, Namibia and USA (Arizona). |
Sodalite
 Sodalite is named in reference to the sodium content it has. It is found in light to dark pure blue color and is well known in the semi-precious stone world. It is the only feldspathoid which contains chlorine. Sodalite is named in reference to the sodium content it has. It is found in light to dark pure blue color and is well known in the semi-precious stone world. It is the only feldspathoid which contains chlorine.
Sodalite balances the mental and emotional bodies. It cleanses the aura, soothes and calms inflammations. It fuels a person’s creative processes and also enhances wisdom. It helps to make clear and rational decisions. It builds self confidence, helps fight lymphatic cancer and also boost the immune system.
Sodalite is found in Namibia, Brazil, Canada and the USA. The hardness is 6 and specific gravity, 2.13 – 2.29.
Gemstones: Lapis Lazuli







Lapis Lazuli is a gemstone compared to stars in the sky.
Stone’s names: Lapis Lazuli, Lazurite.
Color: Lapis Lazuli occurs in various shades of blue with some qualities being speckled with white calcite and some with yellow pyrite. The finest Lapis Lazuli is even blue color with little or no veining from other elements.
Description: Lapis lazuli is a semiprecious stone valued for its deep blue color. The source of the pigment ultramarine, Lapis lazuli is not a mineral but a rock colored by lazurite. In addition to the sodalite minerals in lapis lazuli, small amounts of white calcite and of pyrite crystals are usually present.
Because lapis is a rock of varying composition, its physical properties are variable.
The name’s origin: The name lapis comes from word pencil in Spanish.
Wedding anniversary: Lapis Lazuli is the anniversary gemstone for the 7th and 9th year of marriage.
Care and treatment: Lapis Lazuli can easily be scratched or chipped. Water can dissolve the stone’s protective coatings, hence clean your lapis lazuli jewelry with a soft dry cloth.
From the stone history: Lapis Lazuli with deep azure blue color, often flecked with golden pyriteinclusions, was treasured by ancient Babylonian and Egyptian civilizations and often worn by royalty. Lapis lazuli was widely used by Egyptians for cosmetics and painting . Persian legend says that the heavens owed their blue color to a massive slab of Lapis upon which the earth rested. Lapis Lazuli was believed to be a sacred stone, buried with the dead to protect and guide them in the afterlife.
Lapis lazili is one of the gemstones, that used incommesso, also called florentine mosaic. Commesso is a technique of fashioning pictures with thin, cut-to-shape pieces of brightly colored, semiprecious stones, developed in Florence in the late 16th century. The stones most commonly used are agates, quartzes,chalcedonies,jaspers, granites, porphyries, petrified woods, and lapis lazuli. Commesso pictures, used mainly for tabletops and small wall panels, range from emblematic and floral subjects to landscapes.
Visit all-that-gifts.com – the online store that offer a large collection of pictures decorated with natural precious and semiprecious stones.
For centuries Lapis Lazuli has been prized for jewelry. But it has also been used to make the beautiful blue paint ultramarine and has been used as a source of writing instruments. Ultramarine is used in paints, lacquers, and decorating materials. It has a particularly brilliant blue color and is very lightfast.
Shopping guide: Lapis lazuli has been widely used as a semiprecious stone throughout history. It is most often seen as a necklace of beads or carved pendants.
Fine natural Lapis Lazuli can be rather pricey. Jewelry with the high quality stones with no calcite or pyriteveins can be quite expensive. Much of the jewelry that is sold as Lapis is an artificially colored jasper from Germany that shows colorless specks of clear, crystallized quartz and never the goldlike flecks of pyrite.
Healing ability: The stone is said to increase psychic abilities. Lapis is said to be a cure for melancholy and for certain types of fever. Lapis lazuli eliminates negative emotions. It relieves sore throat pain.
Mystical power: Traditionally believed to increase mental clarity, virility, and calm. Lapis Lazuli is energy focuser for teachers, lecturers and speakers. Enhances creative self-expression. It is believed to be useful in relieving depression and promoting spirituality. Lapis Lazuli is also powerful during meditation.
Deposits: The main supplies of Lapis Lazuli are found in the Afghanistan, Egypt, Canada, Chile, the US, and South America. The most important sources are the mines in Badakhshan, northeastern Afghanistan, and near Ovalle, Chile, where gemstone is usually pale rather than deep blue.
Dumortierite
Dumortierite is a fibrous variably colored aluminium boro-silicate mineral, Al6.5-7BO3(SiO4)3(O,OH)3. Dumortierite crystallizes in theorthorhombic system typically forming fibrous aggregates of slender prismatic crystals. The crystals are vitreous and vary in color from brown, blue, and green to more rare violet and pink. Substitution of iron and other tri-valent elements for aluminium result in the color variations. It has a Mohs hardness of 7 and aspecific gravity of 3.3 to 3.4. Crystals show pleochroism from red to blue to violet.Dumortierite quartz is blue colored quartz containing abundant dumortierite inclusions.
Dumortierite was first described in 1881 for an occurrence in Chaponost, in the Rhône-Alps ofFrance and named for the Frenchpaleontologist Eugene Dumortier (1803-1873). It typically occurs in high temperature aluminium rich regional metamorphic rocks, those resulting fromcontact metamorphism and also in boron rich pegmatites. The most extensive investigation on dumortierite was done on samples from the high grade metamorphic Gfohl unit in Austria by Fuchs et al. (2005).
It is used in the manufacture of high grade porcelain. It is sometimes mistaken for sodalite and has been used as imitation lapis lazuli.
Sources of Dumortierite include Austria, Canada, France, Italy, Madagascar, Namibia,Nevada, Norway, Poland, Russia and Sri Lanka |
Benitoite
Benitoite is a rare blue barium titanium silicate mineral, found in hydrothermally alteredserpentinite. Benitoite fluoresces under short wave ultraviolet light, appearing light blue in color.
It was first described in 1907 by George D. Louderback, who named it benitoite, “as it occurs near the head waters of the San Benito River in San Benito County,” California.[3][4]
Uses of benitoite
Benitoite’s main uses are as collector’s specimens, especially in specimens which show off this mineral’s unique crystals, or specimens in which benitoite occurs with its commonly associated minerals. Benitoite’s hardness also makes it suitable for use as a gemstone, though the general lack of usable material has limited this use.
Associated minerals and locations
Benitoite typically occurs with an unusual set of minerals, along with minerals that make up its host rock. Frequently associated minerals include:

Blue Benitoite Crystals on white natrolite, Dallas Gem Mine, San Benito Co., California, USA
Benitoite is a rare mineral found in very few locations including San Benito County, California,Japan and Arkansas. In the San Benito occurrence it is found in natrolite veins withinglaucophane schist within a serpentinite body. In Japan it occurs in a magnesio-riebeckite-quartz-phlogopite-albite dike cutting a serpentinite body.[5] Benitoite is typically found with some combination of natrolite, joaquinite, and neptunite on a greenish-grey serpentinite base.
Benitoite is the official state gem of California.
Prehnite
Prehnite is a phyllosilicate of calcium and aluminium with the formula: Ca2Al(AlSi3O10)(OH)2. Limited Fe3+ substitutes for aluminium in the structure. Prehnite crystallizes in theorthorhombic crystal system. It is brittle with an uneven fracture and a vitreous to pearly lustre. Its hardness is 6-6.5, its specific gravity is 2.80-2.90 and its color varies from light green to blue or white. It is translucent.
Though not a zeolite, it is found associated with them and with datolite, calcite, etc. in veins and cavities of basaltic rocks, sometimes in granites, syenites, or gneisses. It is an indicator mineral of the prehnite-pumpellyite metamorphic facies. It was first described in 1789 for an occurrence in Haslach, Harzburg and Oberstein, Germany, and named for Colonel Hendrik Von Prehn (1733-1785), an early Dutch governor of the Cape of Good Hope colony.[1] |
Diopside
Diopside is a monoclinic pyroxene mineral with composition MgCaSi2O6. It forms complete solid solution series with hedenbergite(FeCaSi2O6) and augite, and partial solid solutions withorthopyroxene and pigeonite. It forms variably colored, but typically dull green crystals in themonoclinic prismatic class. It has two distinct prismatic cleavages at 87 and 93° typical of the pyroxene series. It has a Mohs hardness of six, a Vickers hardness of 7.7 GPa at a load of 0.98 N[3], and a specific gravity of 3.25 to 3.55. It is transparent to translucent with indices of refraction of nα=1.663–1.699, nβ=1.671–1.705, and nγ=1.693–1.728. The optic angle is 58° to 63°.
Diopside is found in ultramafic (kimberlite and peridotite) igneous rocks, and diopside-rich augite is common in mafic rocks, such as olivine basalt and andesite. Diopside is also found in a variety of metamorphic rocks, such as in contact metamorphosed skarnsdeveloped from high silica dolomites. It is an important mineral in the Earth‘s mantle and is common inperidotite xenoliths erupted inkimberlite and alkali basalt.
Diopside is a precursor of chrysotile (white asbestos) by hydrothermal alteration and magmatic differentiation;[4] it can react with hydrous solutions of magnesium and chlorine to yield chrysotile by heating at 600°C for three days.[5] Some vermiculite deposits, most notably those in Libby, Montana, are contaminated with chrysotile (as well as other forms of asbestos) that formed from diopside.[6]
At relatively high temperatures, there is a miscibility gap between diopside and pigeonite, and at lower temperatures, between diopside and orthopyroxene. Thecalcium/(calcium+magnesium+iron) ratio in diopside that formed with one of these other two pyroxenes is particularly sensitive to temperature above 900°C, and compositions of diopside in peridotite xenoliths have been important in reconstructions of temperatures in the Earth’s mantle.
Gemstone quality diopside is found in two forms: the black star diopside and the chrome diopside (which includes chromium giving it a rich green colour). At 5.5–6.5 on the Mohs scale, chrome diopside is relatively soft to scratch. Mohs scale of hardness does not measure tensile strength or resistance to fracture.
Violane is a manganese rich variety of diopside, violet to light blue in colour.[7]
Chrome diopside ((Ca,Na,Mg,Fe,Cr)2(Si,Al)2O6) is a common constituent of peridotitexenoliths, and dispersed grains are found nearkimberlite pipes, and as such are a prospecting indicator for diamonds. Occurrences are reported in Canada, South Africa, Russia and a wide variety of other locations.
Diopside was first described about 1800 and derives its name from the Greek dis, “twise”, andòpsè, “face” in reference to the two ways of orienting the vertical prism.
Amblygonite
| Amblygonite |
 |
| General |
| Category |
Mineral |
| Chemical formula |
(Li,Na)AlPO4(F,OH) |
| Identification |
| Color |
Generally white or creamy, but can also be colorless or pale yellow, green, blue, beige, gray, brown or pink. |
| Crystal habit |
Prismatic to columnar form |
| Crystal system |
Triclinic |
| Twinning |
Microscopic polysynthetic twinning common |
| Cleavage |
[100] Perfect, [110] Good, [011] Distinct |
| Fracture |
Irregular/Uneven,Sub-Conchoidal |
| Mohs Scalehardness |
5.5 – 6[1] |
| Luster |
Vitreous to pearly[1] |
| Specific gravity |
2.98 – 3.11 |
| Polish luster |
greasy to vitreous (in gem material)[1] |
| Optical properties |
Double refractive, biaxial, may be either positive or negative[1] |
| Refractive index |
na=1.577 – 1.591,
nb=1.592 – 1.605,
nc=1.596 – 1.613 |
| Birefringence |
.020 – .027[1] |
| Pleochroism |
weak to none[1] |
| Ultravioletfluorescence |
very weak green in long wave, light blue phosphorescence in long wave and short wave [1] |
Amblygonite is a fluorophosphate mineral, (Li,Na)AlPO4(F,OH), composed of lithium,sodium, aluminium, phosphate, fluoride andhydroxide. The mineral occurs in pegmatitedeposits and is easily mistaken for albite and other feldspars. Its density, cleavage and flame test for lithium are diagnostic. Amblygonite forms a series with montebrasite, the low fluorine endmember. Geologic occurrence is in granite pegmatites, high-temperature tin veins, andgreisens. Amblygonite occurs with spodumene, apatite,lepidolite, tourmaline, and other lithium-bearing minerals in pegmatite veins. It contains about 10% lithium, and has been utilized as a source of lithium. The chief commercial sources have historically been the deposits of California and France.
History
The mineral was first discovered in Saxony by August Breithaupt in 1817, and named by him from the Greek amblus, blunt, andgouia, angle, because of the obtuse angle between the cleavages. Later it was found at Montebras, Creuse, France, and at Hebron inMaine; and because of slight differences in optical character and chemical composition the names montebrasite and hebronite have been applied to the mineral from these localities. It has been discovered in considerable quantity at Pala in San Diego county,California; Caceres, Spain; and the Black Hills of South Dakota. The largest documented single crystal of amblygonite measured 7.62×2.44×1.83 m3 and weighed ~102 tons.[2]
Gemology
Transparent amblygonite has been faceted and used as a gemstone. As a gemstone set into jewelry it is vulnerable to breakage and abrasion from general wear, as its hardness and toughness are poor.[1] The main sources for gem material are Brazil and the U.S..Australia,France, Germany, Namibia, Norway, and Spain have also produced gem quality amblygonite.[1]
Dioptase
Dioptase is an intense emerald-green to bluish-green copper cyclosilicate mineral. It is transparent to translucent. Itsluster is vitreous to sub-adamantine. Its formula is CuSiO3·H2O (also reported as CuSiO2(OH)2). It has a hardness of 5, the same as tooth enamel. It specific gravity is 3..28–3.35, and it has two perfect and one very good cleavage directions. Additionally, dioptase is very fragile and specimens must be handled with great care. It is atrigonal mineral, forming 6-sidedcrystals that are terminated by rhombohedra.
History
Late in the 18th century, copper miners at the Altyn-Tyube (Altyn-Tube) mine, Karagandy Province,Kazakhstan [1] thought they found an emerald deposit of their dreams. They found fantastic cavities in quartz veins in a limestone, filled with thousands of lustrous emerald-green transparent crystals. The crystals were dispatched to Moscow, Russia for analysis. However the mineral’s inferior hardness of 5 compared with emerald’s greater hardness of 8 easily distinguished it. Later Fr.René Just Haüy (the famed French mineralogist) in 1797 determined that the enigmatic Altyn-Tyube mineral was new to science and named it dioptase (Greek,dia, “through” and optima, “vision”), alluding to the mineral’s two cleavage directions that are visible inside unbroken crystals.
Occurrence
Dioptase is a very rare mineral found mostly in desert regions where it forms as a secondary mineral in the oxidized zone of copper sulfide mineral deposits. However, the process of its formation is not simple, the oxidation of copper sulfides should be insufficient to crystallize dioptase as silica is normally minutely soluble in water except at highly alkaline pH. The oxidation of sulfides will generate highly acidic fluids rich in sulfuric acid that should suppress silica solubility. However, in dry climates and with enough time, especially in areas of a mineral deposit where acids are buffered by carbonate, minute quantities of silica may react with dissolved copper forming dioptase and chrysocolla.
The Altyn Tube mine in Kazakhstan still provides handsome specimens; a brownish quartzite host distinguishes its specimens from other localities. The finest specimens of all were found at the Tsumeb Mine in Tsumeb, Namibia. Tsumeb dioptase is wonderfully lustrous and transparent, with its crystal often perched on an attractive snow-white carbonate matrix. Dioptase is also found in the deserts of the southwestern USA. A notable occurrence is the old Mammoth-Saint Anthony Mine near Mammoth, Arizona where small crystals that make fine micromount specimens are found. In addition, many small, pale-green colored crystals of dioptase have come from the Christmas Mine near Hayden, Arizona. Another classic locality for fine specimens is Renéville, Congo-Brazzaville. Finally, an interesting occurrence is the Malpaso Quarry in Argentina. Here tiny bluish-green dioptase is found on and in quartz. It appears at this occurrence, dioptase is primary and has crystallized with quartz, native copper, and malachite.
Use
Dioptase is popular with mineral collectors and it is occasionally cut into small emerald-likegems. Dioptase and chrysocollaare the only relatively common copper silicate minerals. A dioptase gemstone should never be exposed to ultrasoniccleaning or the fragile gem will shatter.
Kyanite
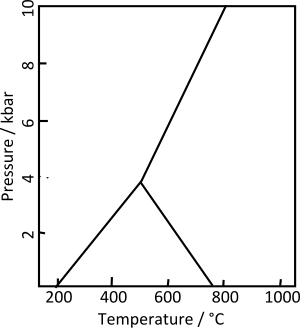
Kyanite, whose name derives from the Greek word kyanos, meaning blue, is a typically blue silicate mineral, commonly found in aluminium-rich metamorphic pegmatites and/orsedimentary rock. Kyanite inmetamorphic rocks generally indicates pressures higher than 4 kilobars. Although potentially stable at lower pressure and low temperature, the activity of water is usually high enough under such conditions that it is replaced by hydrous aluminosilicates such asmuscovite, pyrophyllite, or kaolinite.
Kyanite is a member of the aluminosilicate series, which also includes the polymorphandalusite and the polymorphsillimanite. Kyanite is strongly anisotropic, in that its hardnessvaries depending on its crystallographic direction. While this is a feature of almost all minerals, in kyanite this anisotropism can be considered an identifying characteristic.
At temperatures above 1100 °C, kyanite decomposes into mullite and vitreous silica via the following reaction: 3(Al2O3·SiO2) → 3Al2O3·2SiO2 + SiO2. This transformation results in an expansion.[2]
Uses of kyanite
Kyanite is used primarily in refractory and ceramic products, including porcelain plumbing fixtures and dishware. It is also used in electrical insulators and abrasives. Kyanite has been used as a gemstone, though this use is limited by its anisotropism and perfect cleavage. Kyanite is one of the index minerals that are used to estimate the temperature, depth, and pressure at which a rock undergoes metamorphism. Finally, as with most minerals, kyanite is a collector’s mineral.
Associated minerals
Kyanite is usually found in association with its polymorphs, as well as other silicate minerals. These include:
Alternative names
Kyanite has several alternative names, including disthene, munkrudite and cyanite. White-grey kyanite is also called rhaeticite.
Notes for identification
Kyanite’s elongated, columnar crystals are usually a good first indication of the mineral, as well as its color (when the specimen is blue). Associated minerals are useful as well, especially the presence of the polymorphs or staurolite, which occur frequently with kyanite. However, the most useful characteristic in identifying kyanite is its anisotropism. If one suspects a specimen to be kyanite, verifying that it has two distinctly different hardnesses on perpendicular axes is a key to identification.
Variscite

Cut slab of Variscite at theSmithsonian. Specimen is roughly 0.5 m wide.
Variscite is a hydrated aluminium phosphate mineral (AlPO4·2H2O). It is a relatively rare phosphate mineral. It is sometimes confused with turquoise; however, variscite is usually greener in color.
Variscite is a secondary mineral formed by direct deposition from phosphate-bearing water that has reacted with aluminium-rich rocks in a near-surface environment. It occurs as fine-grained masses in nodules, cavity fillings, and crusts. Variscite often contains white veins of the calcium aluminium phosphate mineral crandallite.
Variscite is sometimes used as a semi-precious stone, and is popular for carvings and ornamental use. It was first described in 1837 and named for the type locality of Variscia, the historical name of Vogtland in Germany. At one time, variscite was called Utahlite. At times, materials which may be turquoise or may be variscite have been marketed as “variquoise”. Appreciation of the color ranges typically found in variscite have made it a popular gem in recent years.[4]
Variscite from Nevada typically contains black spiderwebbing in the matrix and is often confused with green turquoise. Most of the Nevada variscite recovered in recent decades has come from mines located in Lander County.[5]
Notable localities are Lucin, Utah and Fairfield, Utah in the United States. It is also found inGermany, Australia, Poland, Spain[6] andBrazil.

Fluorite
| Fluorite |

Fluorite crystals on display at the Cullen Hall of Gems and Minerals |
| General |
| Category |
mineral |
| Chemical formula |
calcium fluoride CaF2 |
| Identification |
| Color |
Colorless, white, purple, blue, blue-green, green, yellow, brownish-yellow, pink or red |
| Crystal habit |
Occurs as well-formed coarse sized crystals also massive – granular |
| Crystal system |
Isometric, cF12, SpaceGroup Fm-3m, No. 225 |
| Cleavage |
Octahedral…or…[111] Perfect, [111] Perfect, [111] Perfect. |
| Fracture |
Uneven |
| Mohs Scalehardness |
4 |
| Luster |
Vitreous |
| Streak |
White |
| Specific gravity |
3.18 |
| Refractive index |
1.433–1.435 |
| Fusibility |
3 |
| Solubility |
Slightly in water |
| Other characteristics |
sometimesphosphoresceswhen heated or scratched. Other varietiesfluoresce |
Fluorite (also called fluorspar) is a halide mineral composed of calcium fluoride, CaF2. It is an isometricmineral with a cubic habit, though octahedral and more complex isometric forms are not uncommon. Cubic crystals up to 20 cm across have been found at Dalnegorsk, Russia.[1] Crystal twinning is common and adds complexity to the observed crystal habits.
The word fluorite is derived from the Latin root fluo, meaning “to flow” because the mineral has a relatively low melting point and was used as an important flux in smelting. Fluorite gave its name to its constitutive element fluorine.
Occurrence
Fluorite may occur as a vein deposit, especially with metallic minerals, where it often forms a part of thegangue (the worthless “host-rock” in which valuable minerals occur) and may be associated with galena,sphalerite, barite, quartz, and calcite. It is a common mineral in deposits of hydrothermal origin and has been noted as a primary mineral in granites and other igneous rocks and as a common minor constituent ofdolostone and limestone.
Fluorite is a widely occurring mineral which is found in large deposits in many areas. Notable deposits occur in Germany, Austria,Switzerland, England, Norway, Mexico, and Ontario in Canada. Large deposits also occur in Kenya in the Kerio Valley area within the Great Rift Valley. In the United States, deposits are found in Missouri, Oklahoma, Illinois, Kentucky, Colorado, New Mexico,Arizona, Ohio, New Hampshire,New York, Alaska and Texas.. Flourite has been the state mineral of Illinois since 1965. At that time, Illinois was the largest producer of fluorite in the United States; however, the last Illinois mine closed in 1995.
The largest documented single crystal of fluorite was a cube 2.12 m in size and weighed ~16 tons.[2]
Blue John

Vein of Blue John in Treak Cliff Cavern
One of the most famous of the older-known localities of fluorite is Castleton in Derbyshire, England, where, under the name ofDerbyshire Blue John, purple-blue fluorite was extracted from several mines/caves, including the famous Blue John Cavern.. During the 19th century, this attractive fluorite was mined for its ornamental value. The name derives from French “bleu et jaune” (blue and yellow) characterising its color. Blue John is now scarce, and only a few hundred kilograms are mined each year for ornamental andlapidary use. Mining still takes place in the nearby Treak Cliff Cavern.
Recently discovered deposits in China have produced fluorite with coloring and banding similar to the classic Blue John stone.[citation needed]
Fluorescence
Many samples of fluorite fluoresce under ultra-violet light, a property that takes its name from fluorite[3]. Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultra-violet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green and white also occur. The fluorescence of fluorite may be due to impurities such as yttrium or organic matter in the crystal lattice. It is not surprising, therefore, that the color of visible light emitted when a sample of fluorite is fluorescing appears dependent on where the original specimen was collected, different impurities having been included in the crystal lattice in different places. Neither does all fluorite fluoresce equally brightly, even from the same locality. Thereforeultra-violet light is not a reliable tool for the identification of specimens, nor for quantifying the mineral in mixtures. For example, among British fluorites, those from Northumberland, County Durham, and EasternCumbria are the most consistently fluorescent, whereas fluorite from Yorkshire, Derbyshire, and Cornwall, if they fluoresce at all, are generally only feebly fluorescent.
Fluorite also exhibits the property of thermoluminescence.
Color
Fluorite comes in a wide range of colors and has subsequently been dubbed “the most colorful mineral in the world”. The most common colors are purple, blue, green, yellow, or clear. Less common are pink, red, white, brown, black, and nearly every shade in between. Color zoning or banding is commonly present. The color of the fluorite is determined by factors including impurities, exposure to radiation, and the size of the color centers.
Uses
There are three principal types of industrial use for fluorite, corresponding to different grades of purity. Metallurgical grade fluorite, the lowest of the three grades, has traditionally been used as a flux to lower the melting point of raw materials in steel production to aid the removal of impurities, and later in the production of aluminium. Ceramic (intermediate) grade fluorite is used in the manufacture ofopalescent glass, enamelsand cooking utensils. The highest grade, acid grade fluorite, is used to make hydrofluoric acid by decomposing the fluorite with sulfuric acid. Hydrofluoric acid is the primary feedstock for the manufacture of virtually all organic and inorganic fluorine-containing compounds, including fluoropolymers andperfluorocarbons, and is also used to etch glass.
Fluorite is used instead of glass in some high performance telescopes and camera lens elements. Exposure tools for thesemiconductor industry make use of fluorite optical elements for ultraviolet light at 157 nm wavelength. Fluorite has a uniquely high transparency at this wavelength. Fluorite has a very lowdispersion so lenses made from it exhibit less chromatic aberration than those made of ordinary glass. In telescopes it allows crisp images of astronomical objects even at high power. Fluorite also has ornamental and lapidary uses.. Canon Inc. produces synthetic fluorite crystals that are used in their more expensivetelephoto lenses.Nikon has previously manufactured at least one all-fluorite element camera lens (105 mm f/4.5 UV) for the production of ultraviolet images.
Fluorite objective lenses are manufactured by the larger microscope firms (Nikon, Olympus, Carl Zeiss and Leica) due to their strong hexagonal crystal structure most notable for evenly refracting light. Their transparence to ultraviolet light enables them to be used forfluorescence microscopy. The fluorite also serves to correct optical aberrations in these lenses.
Gallery
Fluorite (yellow), calcite (white/grey) and pyrite (gold specs), El Hammon Mine, Morocco
|
Yellow fluorite (~ 4 cm in height)
|
Deep purple cubes of fluorite with galena (gray) and calcite (white) from Illinois, USA
|
Pig carved in fluorite, 5 cm (2 inches) long
|
|
|
Octahedral fluorite crystals from New Mexico, USA
|
Cleaved fluorite octahedra
|
The unit cell of fluorite’s crystal structure
|
|
|
Fluorite with Iron Pyrite and Calcite blooms
|
|
|
|
Chrysocolla
Chrysocolla (hydrated copper silicate) is a mineral, (Cu,Al)2H2Si2O5(OH)4·nH2O. It is of secondary origin and forms in the oxidation zones of copper ore bodies. Associated minerals are quartz, limonite, azurite, malachite, cuprite, and other secondary copper minerals.
Chrysocolla has an attractive blue-green colour and is a minor ore of copper, having ahardness of 2.5 to 3.5. It is also used as an ornamental stone. It is typically found as glassybotryoidal or rounded masses and crusts, or vein fillings. Because of its light color, it is sometimes confused with turquoise. Commonly it occurs only as pourous crusts unsuitable for gem use, but high quality, gem grade chrysocolla can be translucent and is highly prized.
The name comes from the Greek chrysos, “gold”, and kolla, “glue”, in allusion to the name of the material used to solder gold, and was first used by Theophrastus in 315 BCE.
Notable occurrences include Israel, Democratic Republic of Congo, Chile, Cornwall inEngland, and Arizona, Utah, New Mexico and Pennsylvania in the United States. |
Cerussite
Cerussite (also known as lead carbonate or white lead ore) is a mineral consisting of leadcarbonate (PbCO3), and an importantore of lead. The name is from the Latin cerussa, white lead. Cerussa nativa was mentioned by Conrad Gessner in 1565, and in 1832F. S. Beudantapplied the name cruse to the mineral, whilst the present form, cerussite, is due to W. Haidinger (1845). Miners’ names in early use were lead-spar and white-lead-ore.
Cerussite crystallizes in the orthorhombic system and is isomorphous with aragonite. Like aragonite it is very frequently twinned, the compound crystals being pseudo-hexagonal in form. Three crystals are usually twinned together on two faces of the prism, producing six-rayed stellate groups with the individual crystals intercrossing at angles of nearly 60°. Crystals are of frequent occurrence and they usually have very bright and smooth faces. The mineral also occurs in compact granular masses, and sometimes in fibrous forms. The mineral is usually colorless or white, sometimes grey or greenish in tint and varies from transparent to translucent with an adamantine lustre. It is very brittle, and has a conchoidal fracture. It has aMohs hardness of 3 to 3.75 and a specific gravity of 6.5. A variety containing 7 % of zinc carbonate, replacing lead carbonate, is known as iglesiasite, from Iglesias in Sardinia, where it is found.
The mineral may be readily recognized by its characteristic twinning, in conjunction with the adamantine lustre and high specific gravity. It dissolves with effervescence in dilute nitric acid. A blowpipe test will cause it to fuse very readily, and gives indications for lead.
Finely crystallized specimens have been obtained from the Friedrichssegen mine in Lahnsteinnear Nassau, Johanngeorgenstadt inSaxony, Mies in Bohemia, Phoenixville in Pennsylvania,Broken Hill, New South Wales; and several other localities. Delicate acicular crystals of considerable length were found long ago in the Pentire Glaze mine near St Minver in Cornwall. It is often found in considerable quantities, and contains as much as 77.5% of lead.
Lead(II) carbonate is practically insoluble in neutral water (solubility product [Pb2+][CO32-] ≈ 1.5×10-13 at 25 °C), but will dissolve in dilute acids.
Commercial uses
“White lead” is the key ingredient in (now discontinued) lead paints. Ingestion of lead-based paint chips is the most common cause of lead poisoning in children.[3]
Both “white lead” and lead acetate have been used in cosmetics throughout history, though this practice has ceased in Western countries.[4]
Serpentine
The serpentine group describes a group of common rock-forming hydrous magnesium ironphyllosilicate ((Mg,Fe)3Si2O5(OH)4) minerals; they may contain minor amounts of other elements including chromium, manganese, cobalt andnickel. In mineralogy and gemology, serpentine may refer to any of 20 varieties belonging to the serpentine group. Owing to admixture, these varieties are not always easy to individualize, and distinctions are not usually made. There are three important mineral polymorphs of serpentine: antigorite, chrysotile andlizardite.
Overview
“Their olive green color and smooth or scaly appearance is the basis of the name from the Latin serpentinus, meaning serpent rock,” according to Best (2003). They have their origins inmetamorphic alterations of peridotite and pyroxene. Serpentines may also pseudomorphouslyreplace other magnesium silicates. Alterations may be incomplete, causing physical properties of serpentines to vary widely. Where they form a significant part of the land surface, the soil is unusually high in clay.
Antigorite is the polymorph of serpentine that most commonly forms during metamorphism of wet ultramafic rocks and is stable at the highest temperatures—to over 600°C at depths of 60 km or so. In contrast, lizardite and chrysotile typically form near the Earth’s surface and break down at relatively low temperatures, probably well below 400°C. It has been suggested that chrysotile is never stable relative to either of the other two serpentine polymorphs.
Samples of the oceanic crust and uppermost mantle from ocean basins document thatultramafic rocks there commonly contain abundant serpentine. Antigorite contains water in its structure, about 13 percent by weight. Hence, antigorite may play an important role in the transport of water into the earth in subduction zones and in the subsequent release of water to create magmas in island arcs, and some of the water may be carried to yet greater depths.
Soils derived from serpentine are toxic to many plants, because of high levels of nickel,chromium, and cobalt; growth of many plants is also inhibited by low levels of potassium andphosphorus and a low ratio of calcium/magnesium. The flora is generally very distinctive, with specialised, slow-growing species. Areas of serpentine-derived soil will show as strips ofshrubland and open, scattered small trees (often conifers) within otherwise forested areas; these areas are calledserpentine barrens.
Most serpentines are opaque to translucent, light (specific gravity between 2.2–2.9), soft (hardness 2.5–4), infusible and susceptible to acids. All are microcrystalline and massive inhabit, never being found as single crystals. Luster may be vitreous, greasy or silky. Colours range from white to grey, yellow to green, and brown to black, and are often splotchy or veined. Many are intergrown with other minerals, such as calcite and dolomite. Occurrence is worldwide; New Caledonia,Canada (Quebec), USA (northern California), Afghanistan,Cornwall, China, Asia, France, Norway and Italy are notable localities.
Rock composed primarily of these minerals is called serpentinite. Serpentines find use in industry for a number of purposes, such as railway ballasts, building materials, and the asbestiform types find use as thermal and electrical insulation (chrysotile asbestos). The asbestos content can be released to the air when serpentine is excavated and if it is used as a road surface, forming a long term health hazard by breathing. Asbestos from serpentine can also appear at low levels in water supplies through normal weathering processes, but there is as yet no identified health hazard associated with use or ingestion. In its natural state, some forms of serpentine react with carbon dioxide and re-release oxygen into the atmosphere.
The more attractive and durable varieties (all of antigorite) are termed “noble” or “precious” serpentine and are used extensively as gems and in ornamental carvings. Often dyed, they may imitate jade. Misleading synonyms for this material include “Korean jade”, “Suzhou jade”, “Styrian jade”, and “New jade”. New Caledonian serpentine is particularly rich in nickel, and is the source of most of the world’s nickel ore.

Polished slab of bowenite serpentine, a variety of antigorite. Typical cloudy patches and veining are apparent.
The Māori of New Zealand once carved beautiful objects from local serpentine, which they called tangiwai, meaning “tears”. Material quarried in Afghanistan, known as sang-i-yashm, has been used for generations. It is easily carved, taking a good polish, and is said to have a pleasingly greasy feel.
The lapis atracius of the Romans, now known as verde antique, or verde antico, is a serpentinite breccia popular as a decorative facing stone. In classical times it was mined atCasambala, Thessaly, Greece. Serpentinite marbles are also widely used: GreenConnemara marble (or Irish green marble) from Connemara, Ireland (and many other sources), and redRosso di Levanto marble from Italy. Use is limited to indoor settings as serpentinites do not weather well.
Antigorite
Lamellated antigorite occurs in tough, pleated masses. It is usually dark green in color, but may also be yellowish, gray, brown or black. It has a hardness of 3.5–4 and its luster is greasy. The monoclinic crystals show micaceous cleavage and fuse with difficulty. Antigorite is named after its type locality, the Valle di Antigorio in Italy.
Bowenite is an especially hard serpentine (5.5) of a light to dark apple green color, often mottled with cloudy white patches and darker veining. It is the serpentine most frequently encountered in carving and jewellery. The name retinalite is sometimes applied to yellow bowenite. The New Zealand material is called tangiwai.
Although not an official species, bowenite is the state mineral of Rhode Island: this is also the variety’s type locality. A bowenite cabochon featured as part of the “Our Mineral Heritage Brooch”, was presented to First Lady Mrs. Lady Bird Johnson in 1967.
Williamsite is a local varietal name for antigorite that is oil-green with black crystals ofchromite or magnetite often included. Somewhat resembling fine jade, williamsite is cut into cabochons and beads. It is found mainly in Maryland andPennsylvania, USA.[1]
Lizardite
Extremely fine-grained, scaly lizardite (also called orthoantigorite) comprises much of the serpentine present in serpentine marbles. It is triclinic, has one direction of perfect cleavage, and may be white, yellow or green. Lizardite is translucent, soft (hardness 2.5) and has an average specific gravity of 2.57. It can be pseudomorphous after enstatite,olivine or pyroxene, in which case the name bastite is sometimes applied. Bastite may have a silky lustre.
Lizardite is named after its type locality on the Lizard Peninsula, Cornwall, UK.[2] It is worked by local artisans into various trinkets which are sold to tourists.
The California State Rock is Serpentinite.
Ulexite

A fragment of ulexite displaying characteristic optical property
Ulexite (NaCaB5O9·8H2O) (hydrated sodium calcium borate hydroxide) is a mineral occurring in silky white rounded crystalline masses or in parallel fibers. It was named after the German chemist Georg Ludwig Ulex (1811−1883) who first discovered it.
Ulexite is a structurally complex mineral, with a basic structure containing chains of sodium, water and hydroxide octahedra. The chains are linked together by calcium, water, hydroxide and oxygen polyhedra and massive boron units. The boron units have a formula of B5O6(OH)6and a charge of -3, and are composed of three borate tetrahedra and two borate triangular groups. Hardness is 2 (softer than a fingernail) and specific gravity is approximately 1.97.[1]
Ulexite is found with the mineral borax and is directly deposited in arid regions from the evaporation of water in intermittent lakes called playas. The precipitated ulexite commonly forms a “cotton ball” tuft of acicular crystals. Ulexite is also found in a vein-like bedding habit composed of closely-packed fibrous crystals, also known as “TV rock” or “TV stone” due to its unusual optical characteristics. The fibers of TV rock act as fiber optics, transmitting light along their lengths by internal reflection, and when a piece of TV rock is cut with flat polished faces perpendicular to the orientation of the fibers a good-quality specimen will display an image of whatever surface is adjacent to its other side (as shown in the photograph).
The fiber-optic effect is the result of the polarization of light into slow and fast rays within each fiber, the internal reflection of the slow ray, and the refraction of the fast ray into the slow ray of an adjacent fiber. An interesting consequence is the generation of three cones, two of which are polarized, when a laser beam obliquely illuminates the fibers. These cones can be seen when viewing a light source through the mineral.[1]
Ulexite decomposes in hot water. It is found principally in California and Nevada, USA;Tarapaca Region in Chile, and Kazakhstan. |
Howlite
Howlite, a calcium borosilicate hydroxide (Ca2B5SiO9(OH)5), is a silicate mineral found inevaporite deposites.[4] Howlite was discovered at Tick Canyon, California in 1868 by Henry How (1828 – 1879), a Canadian chemist, geologist, and mineralogist.[1][3]
In appearance, it is white with fine grey or black veins in an erratic, often web-like pattern, and is opaque with a sub-vitreous lustre. Its structure is monoclinic with a Mohs hardness of 3.5 and lacks regular cleavage.

Howlite, dyed blue as a turquoise simulant
Howlite is commonly used to make decorative objects such as small carvings or jewelry components. Because of its porous texture, howlite can be easily dyed to imitate other minerals, especially turquoise because of the superficial similarity of the veining patterns. The dyed howlite (or magnesite) is marketed as turquenite.[5] Howlite is also sold in its natural state, sometimes under the misleading trade names of “white turquoise” or “white buffalo turquoise”, or the derived name “white buffalo stone”. |
Onyx
Onyx is a cryptocrystalline form of quartz. The colors of its bands range from white to almost every color (save some shades, such as purple or blue). Commonly, specimens of onyx available contain bands of colors of white, tan, and brown.Sardonyx is a variant in which the colored bands are sard (shades of red) rather than black. Pure black onyx is common, and perhaps the most famous variety, but not as common as onyx with banded colors.

Sardonyx (banded agate). The specimen is 2.5 cm (1 inch) wide.
It is usually cut as a cabochon, or into beads, and is also used for intaglios and cameos, where the bands make the image contrast with the ground. Some onyx is natural but much is produced by the staining of agate.
The name has sometimes been used, incorrectly, to label other banded lapidary materials, such as banded calcite found inMexico, Pakistan, and other places, and often carved, polished and sold. This material is much softer than true onyx, and much more readily available. The majority of carved items sold as ‘onyx’ today are this carbonate material.[1]
Etymology
Onyx comes through Latin from the Greek onyx meaning ‘claw’ or ‘fingernail’. With its fleshtone color, onyx can be said to resemble a fingernail. The English word ‘nail’ is cognatewith the Greek word.[2]
Historical usage
The Beinecke Rare Book and Manuscript Library at Yale University was originally planned to be coated in green onyx. However, there wasn’t sufficient green onyx in the world to build such a structure, so that the designers used marble. Onyx was known to the ancient Greeks and Romans.[3] Use of sardonyx appears in the art of Minoan Crete, notably from the archaeological recoveries at Knossos.[4] Onyx was used in Egypt as early as the Second Dynasty to make bowls and other pottery items.[5] It is also mentioned in Exodus 25.

Black onyx with bands of colors.
|
Tufa
Not to be confused with tuff, a hard volcanic rock that is sometimes called tufa. This article is about the geological formation. For the historic Chinese name, see Southern Liang. Tufa (sometimes also known as sinter) is a soft, friable and porous calcite rock. It is acalcium carbonate (CaCO3) deposit that forms by chemical/biological precipitation from bodies of water with a high dissolved calcium content. Calcareous tufa is not to be confused with tuff, a hard volcanic rock that is also sometimes called tufa.
Tufa deposition occurs in seven known ways:
- Mechanical precipitation by wave action against the shore. This form of tufa can be useful for identifying the shoreline of extinct lakes (for example in the Lake Lahontanregion).
- Precipitation from supersaturated hot spring water entering cooler lake water.
- Precipitation in lake bottom sediments which are fed by hot springs from below.
- Precipitation from calcium-bearing spring water flowing into an alkaline lake.
- Precipitation throughout a lake as the lake water evaporates, leaving the lake supersaturated in calcium.
- Through the agency of algae. Microbial influence is often vital to tufa precipitation and may be involved in the other methods listed.
- Precipitation from cold water springs (for example in the foothills of the Rocky Mountains near Hinton, Alberta).
Tufa is common in many parts of the world. There are some prominent towers of tufa at Mono Lake and Trona Pinnacles in California, USA, formed by the fourth method mentioned above whilst submerged and subsequently exposed by falling water levels. Tufa is also common inArmenia and Great Britain.
Practical use
Tufa is today occasionally shaped into a planter. Its porous consistency makes tufa ideal foralpine gardens. A concrete mixture called hypertufa is used for similar purposes.
Orbicular jasper

Orbicular jasper from Madagascar
Orbicular jasper is a variety of jasper which contains variably-colored orbs or spherical inclusions or zones. In highly silicifiedrhyolite or tuff, quartz and feldspar crystallize in radial aggregates of needle-like crystals which provide the basis or seed for the orbicular structure seen in this kind of jasper[1]. The material is quite attractive when polished and is used as an ornamental stone orgemstone.
Various local or commercial names have been used for the material, such as kinradite, oregonite, owyhee jasper, ocean jasper and poppy-patterned jasper, depending on the source.Poppy-patterned jasper or Poppy jasper is the varietal name for material fromMorgan Hill,Santa Clara County, California. The trade name ocean jasper is used for a variety found along the intertidal shores of northeast Madagascar. In Nebraska orbicular jasper is found in altered rhyolite beds noted for a variety of jaspers and related agates.
Alabaster

An uplighter lamp made from Italian alabaster (white and brown types). The base is 5 inches (13 cm) in diameter

Alabaster vase of Tutankhamun
Alabaster is a name applied to varieties of two distinct minerals: gypsum (a hydrous sulfate ofcalcium) and calcite (a carbonate of calcium). The former is the alabaster of the present day; the latter is generally the alabaster of the ancients.
The two kinds are readily distinguished from one another by their relative hardness. The gypsum kind is so soft as to be readily scratched by a fingernail (Mohs hardness 1.5 to 2), while the calcite kind is too hard to be scratched in this way (Mohs hardness 3), though it does yield readily to a knife. Moreover, the calcite alabaster, being a carbonate, effervesces on being touched withhydrochloric acid, whereas the gypsum alabaster, when so treated, remains practically unaffected.
Due to the characteristic color of white alabaster, the term has entered the vernacular as ametonym for white things, particularly “alabaster skin”. The usage as whiteness also occurs in a line from the poem and song, America the Beautiful.
Etymology
The origin of alabaster is in Middle English, through Old French alabastre, in turn derived fromLatin alabaster and that from Greekαλάβαστρος (alabastros) or αλάβαστος (alabastos), the latter being the word for a vase made of alabaster.[1] This may further derive from the ancient Egyptian word a-labaste (vessel of the Egyptian goddess Bast).[2][3] It has been suggested that the name was derived from the town of Alabastron in Egypt, while an Arabic etymological origin has also been suggested.[4]
Types
Calcite alabaster
This substance, the “alabaster” of the Bible, is often termed Oriental alabaster, since the early examples came from the Far East. The Greek name alabastrites is said to be derived from the town of Alabastron, in Egypt, where the stone was quarried, but the locality probably owed its name to the mineral; the origin of the mineral name is obscure. This “Oriental” alabaster was highly esteemed for making small perfume bottles or ointment vases called alabastra, and this has been conjectured to be a possible source of the name. Alabaster was also employed in Egypt for canopic jars and various other sacred and sepulchral objects. A splendidsarcophagus, sculptured in a single block of translucent calcite alabaster from Alabastron, is in the Sir John Soane’s Museum, London. This was discovered by Giovanni Belzoni in 1817 in the tomb of Seti I near Thebes. It was purchased by Sir John Soane, having previously been offered to the British Museum..
When cut in thin sheets, alabaster is translucent enough to be used for small windows, and has been used so in medieval churches, especially in Italy. Large alabaster sheets are used extensively in the Cathedral of Our Lady of the Angels (dedicated 2002) of theLos Angeles (California) Archdiocese. The cathedral incorporates special cooling to prevent the panes from overheating and turning opaque.
Calcite alabaster is either a stalagmitic deposit, from the floor and walls of limestone caverns, or a kind of travertine, similarly deposited in springs of calcareous water. Its deposition in successive layers gives rise to the banded appearance that the marble often shows on cross-section, whence it is known as onyx-marble or alabaster-onyx, or sometimes simply as onyx— a term which should, however, be restricted to siliceous minerals. Egyptian alabaster has been extensively worked near Suez and near Assiut; there are many ancient quarries in the hills overlooking the plain of Tell el Amarna. The Algerian onyx-marble has been largely quarried in the province of Oran. In Mexico, there are famous deposits of a delicate green variety at La Pedrara, in the district ofTecali, near Puebla.. Onyx-marble occurs also in the district of Tehuacán and at several localities in California, Arizona, Utah,Colorado andVirginia.
Gypsum alabaster

Statue made of Alabaster,Yemen
When the term “alabaster” is used without any qualification, it invariably means a fine-grainedvariety of gypsum. This mineral, or alabaster proper, occurs in England. However, thousands of gypsum alabaster artifacts dating to the late 4th millennium BC have been found in Tell Brak(present day Nagar), in Syria.[5] And in Mesopotamia, a gypsum alabaster sculpture, believed to represent the god Abu, dates to the first half of the 3rd millennium BC.[6]
Mineral alabaster occurs in England in the Keuper marls of the Midlands, especially atChellaston in Derbyshire, at Fauld inStaffordshire and near Newark in Nottinghamshire. All these localities have been extensively worked. In the 15th century its carving into icons andaltarpieces was a valuable local industry in Nottingham, as well as a major English export. Besides examples of these still in Britain (especially at the Nottingham Castle Museum,British Museum and Victoria and Albert Museum), that trade in itself (rather than just the antiques trade) has scattered examples as far afield as the Musée de Cluny, Spain and Scandinavia.
Alabaster is also found, though in subordinate quantity, at Watchet in Somerset, near Penarthin Glamorganshire, and elsewhere. InCumbria it occurs largely in the New Red rocks, but at a lower geological horizon. The alabaster of Nottinghamshire and Derbyshire is found in thick nodular beds or “floors” in spheroidal masses known as “balls” or “bowls,” and in smaller lenticular masses termed “cakes.” At Chellaston, where the alabaster is known as “Patrick,” it has been worked into ornaments under the name of “Derbyshire spar” — a term more properly applied to fluorspar.
Black alabaster
Black Alabaster is a rare form of the gypsum-based mineral found in only three veins in the world, one each in Oklahoma (USA),Italy, and the People’s Republic of China.
Alabaster Caverns State Park, near Freedom, Oklahoma is home to a natural gypsum cave in which much of the gypsum is in the form of alabaster. There are several types of alabaster found at the site, including pink, white, and the rare black alabaster.
Uses

This alabaster sculpture is untreated: its translucency and satin lustre are preserved. Its base is of marble.
The finer kinds of alabaster are largely employed as an ornamental stone, especially forecclesiastical decoration and for the rails of staircases and halls. Its softness enables it to be readily carved into elaborate forms, but its solubility in water renders it unsuitable for outdoor work. The purest alabaster is a snow-white material of fine tiniforni grain, but it is often associated with an oxide of iron, which produces brown clouding and veining in the stone. The coarser varieties of alabaster are converted by calcination into plaster of Paris, whence they are sometimes known as “plaster stone.”
On the continent of Europe, the centre of the alabaster trade is Florence, Italy. Tuscanalabaster occurs in nodular masses embedded in limestone, interstratified with marls ofMiocene and Pliocene age. The mineral is largely worked by means of underground galleries, in the district of Volterra. Several varieties are recognized — veined, spotted, clouded, agatiform, and others. The finest kind, obtained principally from Castellina, is sent to Florence for figure-sculpture, while the common kinds are carved at a very cheap rate locally into vases, clock-cases and various ornamental objects, in which a large trade is carried on, especially in Florence, Pisa and Livorno.
In order to diminish the translucency of the alabaster and to produce an opacity suggestive of true marble, the statues are immersed in a bath of water and gradually heated nearly to the boiling-point — an operation requiring great care, for if the temperature is not carefully regulated, the stone acquires a dead-white, chalky appearance. The effect of heating appears to be a partial dehydration of the gypsum. If properly treated, it very closely resembles true marble and is known as marmo di Castellina. Sulphate of lime (gypsum) was used also by the ancients, and was employed, for instance, in Assyrian sculpture, so that some of the ancient alabaster is identical with the modern stone.
Alabaster may be stained by digesting it, after being heated in various pigmentary solutions. In this way a good imitation of coral has been produced (alabaster coral). |
Coral
Corals are marine organisms from the class Anthozoa and exist as small sea anemone-like polyps, typically in colonies of many identical individuals. The group includes the important reef builders that are found in tropical oceans, which secretecalcium carbonate to form a hard skeleton.
A coral “head”, commonly perceived to be a single organism, is formed from myriads of individual but genetically identicalpolyps, each polyp only a few millimeters in diameter. Over thousands of generations, the polyps lay down a skeleton that is characteristic of their species. An individual head of coral grows by asexual reproduction of the individual polyps. Corals also breed sexually by spawning, with corals of the same species releasing gametes simultaneously over a period of one to several nights around a full moon.
Although corals can catch small fish and animals such as plankton using stinging cells on their tentacles, these animals obtain most of their nutrients from photosynthetic unicellular algae called zooxanthellae. Consequently, most corals depend on sunlight and grow in clear and shallow water, typically at depths shallower than 60 m (200 ft). These corals can be major contributors to the physical structure of the coral reefs that develop in tropical and subtropical waters, such as the enormous Great Barrier Reef off the coast of Queensland, Australia. Other corals do not have associated algae and can live in much deeper water, with the cold-water genus Lophelia surviving as deep as 3000 m.[3] Examples of these can be found living on the Darwin Mounds located north-west of Cape Wrath, Scotland. Corals have also been found off the coast ofWashington State and the Aleutian Islands in Alaska.
Corals coordinate behaviour by communicating with each other.[4]
Phylogeny
Corals belong to the class Anthozoa and are divided into two subclasses, depending on the number of tentacles or lines of symmetry, and a series of orders corresponding to their exoskeleton, nematocyst type and mitochondrial genetic analysis.[1][2][5] Those with eight tentacles are called octocorallia or Alcyonariaand comprise soft corals, sea fans and sea pens. Those with more than eight in a multiple of six are called hexacorallia or Zoantharia. This group includes reef-building corals (Scleractinians), sea anemones andzoanthids.
Anatomy
While a coral head appears to be a single organism, it is actually a head of many individual, yet genetically identical, polyps. The polyps are multicellular organisms that feed on a variety of small organisms, from microscopic plankton to small fish.
Polyps are usually a few millimeters in diameter, and are formed by a layer of outer epithelium and inner jellylike tissue known as the mesoglea. They are radially symmetrical with tentacles surrounding a central mouth, the only opening to the stomach or coelenteron, through which both food is ingested and waste expelled.
The stomach closes at the base of the polyp, where the epithelium produces an exoskeleton called the basal plate or calicle (L. small cup). This is formed by a thickened calciferous ring (annular thickening) with six supporting radial ridges (as shown below). These structures grow vertically and project into the base of the polyp. When polyps are physically stressed, they contract into the calyx so that virtually no part is exposed above the skeletal platform. This protects the organism from predators and the elements (Barnes, R.D., 1987; Sumich, 1996).[6][7]
The polyp grows by extension of vertical calices which are occasionally septated to form a new, higher, basal plate. Over many generations this extension forms the large calciferous (Calcium containing) structures of corals and ultimately coral reefs.
Formation of the calciferous exoskeleton involves deposition of the mineral aragonite by the polyps fromcalcium ions they acquire from seawater. The rate of deposition, while varying greatly across species and environmental conditions, can be as much as 10 g / m² of polyp / day (0.3 ounce / sq yd / day). This is light dependent, with night-time production 90% lower than that during the middle of the day.[8]

Nematocyst discharge: A dormant nematocyst discharges response to nearby prey touching the cnidocil, the operculum flap opens and its stinging apparatus fires the barb into the prey leaving a hollow filament through which poisons are injected to immobilise the prey, then the tentacles manoeuvre the prey to the mouth.
The polyp’s tentacles trap prey using stinging cells called nematocysts. These are cells modified to capture and immobilize prey, such as plankton, by injecting poisons, firing very rapidly in response to contact. These poisons are usually weak but in fire corals are potent enough to harm humans. Nematocysts can also be found in jellyfish and sea anemones. The toxins injected by nematocysts immobilize or kill prey, which can then be drawn into the polyp’s stomach by the tentacles through a contractile band of epithelium called the pharynx.
The polyps interconnect by a complex and well developed system of gastrovascular canals allowing significant sharing of nutrients and symbiotes. In soft corals these range in size from 50-500 μm in diameter and to allow transport of both metabolites and cellular components.[9]

Close-up of Montastrea cavernosa polyps. Tentacles are clearly visible.
Aside from feeding on plankton, many corals as well as other cnidarian groups such as sea anemones (e.g.Aiptasia), form asymbiotic relationship with a class of algae, zooxanthellae, of the genus Symbiodinium. The sea anemone Aiptasia, while considered a pest among coral reef aquarium hobbyists, has served as a valuable model organism in the scientific study of cnidarian-algal symbiosis. Typically a polyp harbors one particular species of algae. Via photosynthesis, these provide energy for the coral, and aid in calcification.[10] The algae benefit from a safe environment, and use the carbon dioxide and nitrogenous waste produced by the polyp. Due to the strain the algae can put on the polyp, stress on the coral often triggers ejection of the algae, known on a large scale as coral bleaching, as it is the algae that contribute to the brown coloration of corals; other colors, however, are due to host coral pigments, such as GFPs (green fluorescent protein). Ejecting the algae increases the polyps’ chances of surviving stressful periods – they can regain the algae at a later time. If the stressful conditions persist, the corals eventually die.[11]
Reproduction
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows coral to settle new areas.
Sexual

Life cycles of broadcasters and brooders.
Corals predominantly reproduce sexually, with 25% of hermatypic corals (stony corals) forming single sex (gonochoristic) colonies, whilst the rest are hermaphroditic.[12] About 75% of all hermatypic corals “broadcast spawn” by releasing gametes – eggs and sperm – into the water to spread offspring over large distances. The gametes fuse during fertilisation to form a microscopic larvum called a planula, typically pink and elliptical in shape; a moderately sized coral colony can form several thousands of these larvae per year to overcome the huge odds against formation of a new colony.[13]
The planula swims towards light, exhibiting positive phototaxis, to surface waters where they drift and grow for a time before swimming back down to locate a surface on which it can attach and establish a new colony. At many stages of this process there are high failure rates, and even though millions of gametes are released by each colony very few new colonies form. The time from spawning to settling is usually 2 or 3 days, but can be up to 2 months.[14] The larva grows into a coral polyp and eventually becomes a coral head by asexual budding and growth, creating new polyps.

A male star coral, Montastraea cavernosa, releases sperm into the water.
Corals that do not broadcast their eggs are called brooders, this is the case for most non-stony corals. These corals release sperm but harbour eggs, allowing larger, negatively buoyant, planulae to form which the polyp later releases ready to settle.[10] The larva grows into a coral polyp and eventually becomes a coral head by asexual budding.
Synchronous spawning is very typical on a coral reef and often, even when multiple species are present, all the corals on the reef release gametes the same night. This synchrony is essential so that male and female gametes can meet and form planula. The cues that guide the release are complex, but over the short term involve lunar changes, sunset time, and possibly chemical signalling.[12] Synchronous spawning may have the result of forming coral hybrids and is perhaps involved in coral speciation.[15] In some places the coral spawn can be dramatic, usually occurring at night, where the usually clear water becomes cloudy with gametes.
Corals must rely on environmental cues, varying from species to species, to determine the proper time to release gametes into the water. Corals use two methods for sexual reproduction, which differ in whether the female gametes are released:
- Broadcasters, the majority of which mass spawn, rely heavily on environmental cues, because in contrast to brooders they release both sperm and eggs into the water. The corals use long-term cues such as day length, water temperature, and/or rate of temperature change. The short-term cue is most often the lunar cycle, with the sunset cuing the time of release.[12] About 75% of coral species are broadcasters, the majority of which are hermatypic, or reef-building corals.[12] The positively buoyant gametes float towards the surface where fertilization occurs to produce planulalarvae. The planula larvae swim towards the surface light to enter into currents, where they remain usually for two days, but can be up to three weeks, and in one known case two months,[14] after which they settle and metamorphose into polyps and form colonies.
- Brooders are most often ahermatypic (non-reef building) in areas of high current or wave action. Brooders release only sperm, which is negatively buoyant, and can store unfertilized eggs for weeks, lowering the need for mass synchronous spawning events, which do sometimes occur.[12] After fertilization the corals release planula larvae which are ready to settle.
Asexual

Calices (basal plates) ofOrbicella annularisshowing two methods of multiplication: gemmation (small central calicle) and division (large double calicle).
Within a coral head the genetically identical polyps reproduce asexually to allow colony growth. This is achieved either through gemmation (budding) or through division, both shown in the diagrams of Orbicella annularis. Budding involves a new polyp growing from an adult, whereas division forms two polyps each as large as the original.[13]
- Budding expands the size of a coral colony. It occurs when a new corallite grows out from the adult polyp. As the new polyp grows it produces a coelenteron (stomach), tentacles and a mouth. The distance between the new and adult polyps grows, and with it the coenosarc (the common body of the colony; see coral anatomy). Budding can occur by means of:
- Intra-tentacular budding forms from the oral discs of a polyp, meaning that both polyps are the same size and are within the same ring of tentacles.
- Extra-tentacular budding forms from the base of a polyp, and the new polyp is smaller.
- Longitudinal division begins with broadening of a polyp, which then divides the coelenteron. The mouth divides and new tentacles form. The two “new” polyps must generate their missing body parts and exoskeleton..
- Transversal division occurs when polyps and the exoskeleton divide transversally into two parts. This means that one has the basal disc (bottom) and the other has the oral disc (top). The two new polyps must again generate the missing parts.
- Fission occurs in some corals, especially among the family Fungiidae, where the colony is able to split into two or more colonies during the early stages of their development.
Whole colonies can reproduce asexually through fragmentation or bailout, forming another individual colony with the same genome..
- Polyp bailout occurs when a single polyp abandons the colony and re-establishes on a new substrate to create a new adult colony..
- Fragmentation, involves individuals broken from the colony during storms, or other situations where breaking can occur. The separated individuals can start new coral colonies.
Reefs
The hermatypic, stony corals are often found in coral reefs, large calcium carbonate structures generally found in shallow,tropical water. Reefs are built up from coral skeletons and held together by layers of calcium carbonate produced bycoralline algae. Reefs are extremely diverse marine ecosystems being host to over 4,000 species of fish, massive numbers of cnidarians, mollusks, crustaceans, and many other animals.[16]
Types
Hermatypic corals
Hermatype corals or stony corals build reefs. With the help of zooxanthellae, they convert surplus food to calcium carbonate forming a hard skeleton. Hermatype-species include Scleractinia, Millepora, Tubiporaand Heliopora. [17]
In the Caribbean alone 50 species of uniquely structured hard coral exist. Some of the most well known types being:
Ahermatypic corals
Ahermatypic corals are corals that have no zooxanthellae and can therefore not build the solid skeletons that form reefs. They include Alcyonaceas, as well as some Anthipatharia-species (Black coral, Cirripathes,Antipathes). [17] Ahermatypic corals such as sea whips, sea feathers, and sea pens [18] are also known as soft corals. Unlike stony corals, they are flexible, moving back and forth in the current, and often are perforated, with a lace-like appearance. Their skeletons are made of protein, rather than calcium. Soft corals are somewhat less plentiful (in the Caribbean, twenty species appear) than stony corals.
Evolutionary history

The fossil coral Heliophyllum hallifrom the Devonian period, found inCanada.
Although corals first appeared in the Cambrian period,[19] some 542 million years ago, fossils are extremely rare until theOrdovician period, 100 million years later, when Rugose and Tabulate corals became widespread.
Tabulate corals occur in the limestones and calcareous shales of the Ordovician and Silurian periods, and often form low cushions or branching masses alongside Rugose corals. Their numbers began to decline during the middle of the Silurian period and they finally became extinct at the end of the Permian period, 250 million years ago. The skeletons of Tabulate corals are composed of a form of calcium carbonate known as calcite.
Rugose corals became dominant by the middle of the Silurian period, and became extinct early in theTriassic period. The Rugose corals existed in solitary and colonial forms, and are also composed of calcite.
The Scleractinian corals filled the niche vacated by the extinct Rugose and Tabulate species. Their fossils may be found in small numbers in rocks from the Triassic period, and become relatively common in theJurassic and later periods. Scleractinian skeletons are composed of a form of calcium carbonate known asaragonite.[20] Although they are geologically younger than the Tabulate and Rugose corals, their aragonitic skeleton is less readily preserved, and their fossil record is less complete..
|

|
| Timeline of the major coral fossil record and developments from 650 m.y.a. to present.[21][22] |
|
At certain times in the geological past corals were very abundant. Like modern corals, these ancestors built reefs, some of which now lie as great structures in sedimentary rocks.
Fossils of fellow reef-dwellers algae, sponges, and the remains of many echinoids, brachiopods, bivalves,gastropods, andtrilobites appear along with coral fossils. This makes some corals useful index fossils, enabling geologists to date the age the rocks in which they are found.
Coral fossils are not restricted to reef remnants, and many solitary corals may be found elsewhere, such asCyclocyathus, which occurs in England‘s Gault clay formation.
Environmental influences

A healthy coral reef has a striking level of biodiversity in many forms of marine life.
Corals are highly sensitive to environmental changes. Scientists have predicted that over 50% of the world’scoral reefs may be destroyed by the year 2030;[23] as a result most nations protect them through environmental laws. Algae can overwhelm a coral reef if too many nutrients are present. Coral will also die if the water temperature changes by more than a degree or two beyond its normal range or if the salinity of the water drops. In an early symptom of environmental stress, corals expel their zooxanthellae; without their symbiotic unicellular algae, coral tissues become colorless as they reveal the white of their calcium carbonate skeletons, an event known as coral bleaching.[24]
Many governments now prohibit removal of coral from reefs and use education to inform their populations about reef protection and ecology. However, many other human activities damage reefs, including mooring, fishing, diving, mining and construction.
The narrow niche that coral occupies, and the stony corals‘ reliance on calcium carbonate deposition, means they are susceptible to changes in water pH. The increase in atmospheric carbon dioxide has caused enough dissolution of carbon dioxide to lower the ocean’s pH, in a process known as ocean acidification. Lowered pH reduces the ability of corals to produce calcium carbonate, and at the extreme, can entirely dissolve those skeletons. Without deep and immediate cuts in anthropogenic CO2 emissions, scientists fear that ocean acidification will result in the severe degradation or destruction of coral species and ecosystems.[25]

A section through a coral, dyed to determine growth rate
Climatic variations can cause temperature changes that destroy corals. For example, during the 1997-98 warming event all the hydrozoan Millepora boschmai colonies near Panamá were bleached and died within six years – this species is now thought to be extinct.[26]
Uses
Live corals
Local economies near major coral reefs benefit from an abundance of fish and other marine creatures as a food source. Reefs also provide recreational scuba diving and snorkeling tourism. Unfortunately these activities can also have deleterious effects, such as accidental destruction of coral. Coral is also useful as a protection against hurricanes and other extreme weather.
Live coral is highly sought after in the aquarium trade. Provided the proper ecosystem, live coral makes a stunning addition to any salt water aquarium. Soft corals are considered easier to maintain in captivity than hard corals.[27]
Deep sea bamboo corals (Isididae) may be among the first organisms to display the effects of ocean acidification. They produce growth rings similar to those of tree and can provide a view of changes in the condition in the deep sea over time.[28] Other coral biology research presents the possibility that Isididae corals, because of their potential to mimic biological properties, may be usable as living bone implants and in aquatic cultivation.[29]
Coral as a gemstone
Intensely red coral is sometimes called fire coral (but this is not at all the same thing as fire coral). Red coral is very rare now because of overharvesting due to the great demand for perfect red coral in jewelry-making.
Ancient corals

Tabulate coral (a syringoporid); Boone Limestone (LowerCarboniferous) near Hiwasse, Arkansas. Scale bar is 2.0 cm.
Ancient coral reefs on land are often mined for lime or use as building blocks (“coral rag“). Coral rag is an important local building material in places such as the East African coast.
The annual growth bands in bamboo corals and others allow geologists to construct year-by-year chronologies, a form ofincremental dating, which can provide high-resolution records of past climatic andenvironmental changes usinggeochemical techniques.[30]
Certain species of corals form communities called microatolls. The vertical growth of microatolls is limited by average tidal height. By analyzing the various growth morphologies, microatolls can be used as a low resolution record of patterns of sea level change. Fossilized microatolls can also be dated using radioactive carbon dating. Such methods have been used to used to reconstruct Holocene sea levels.[31]
Gallery
Further images: commons:Category:Coral reefs and commons:Category:Coral
|
|
|
Polyps of Eusmilia fastigiata
|
|
Orange cup coral,Balanophyllia elegans
|
|
Brain coral releasing eggs
|
|
|
Amber

Amber pendants. The ovalpendant is 52 by 32 mm (2 by 1.3 inches).
Amber is fossil tree resin, which is appreciated for its color and beauty. Good quality amber is used for the manufacture of ornamental objects and jewelry. Although not mineralized, it is often classified as a gemstone.
A common misconception is that amber is made of tree sap; it is not. Sap is the fluid that circulates through a plant’s vascular system, while resin is the semi-solid amorphous organic substance secreted in pockets and canals through epithelial cells of the plant.
Because it used to be soft and sticky tree resin, amber can sometimes contain insects and even small vertebrates.
Semi-fossilized resin or sub-fossil amber is known as copal.
Amber occurs in a range of different colors. As well as the usual yellow-orange-brown that is associated with the color “amber”, amber itself can range from a whitish color through a pale lemon yellow, to brown and almost black. Other more uncommon colors include red amber (sometimes known as “cherry amber”), green amber, and even blue amber, which is rare and highly sought after.
A lot of the most highly-prized amber is transparent, in contrast to the very common cloudy amber and opaque amber. Opaque amber contains numerous minute bubbles. This kind of amber is known as “bony amber”, even though it is in fact true amber.
Origin of the term

Wood resin, the ancient source of amber
The English word amber stems from the old Arabic word anbargris or ambergris and refers to an oily, perfumed substance secreted by the sperm whale. Middle English ambre > Old French ambre > Medieval Latin ambra (or ambar). It floats on water and is washed up on the beaches. Due to a confusion of terms (see: Abu Zaid al Hassan from Siraf & Sulaiman the Merchant (851), Silsilat-al-Tawarikh (travels in Asia), it came to be the name for fossil resin, which is also found on beaches, and which is lighter than stone, but not light enough to float.
The presence of insects in amber was noticed by the Pliny the Elder in his Naturalis Historiaand led him to the (correct) theory that at some point, amber had to be in a liquid state to cover the bodies of insects. Hence he gave it the expressive name of succinum orgum-stone, a name that is still in use today to describe succinic acid as well as succinite, a term given to a particular type of amber by James Dwight Dana (see below under Baltic Amber).
The Greek name for amber was ηλεκτρον (Electron) and was connected to the Sun God, one of whose titles was Elector or theAwakener.[1] It is discussed by Theophrastus, possibly the first ever mention of the material, and in the 4th century BC. The modern term electron was coined in 1891 by the Irish physicist George Stoney, using the Greek word for amber (and which was then translated as electrum) because of its electrostatic properties and whilst analyzing elementary charge for the first time. The ending -on, common for all subatomic particles, was used in analogy to the word ion.[2][3]
Heating amber will soften it and eventually it will burn, which is why in Germanic languagesthe word for amber is a literal translation of burn-Stone (In German it is Bernstein, in Dutch it is barnsteen etc.). Heated above 200°C, amber suffers decomposition, yielding an “oil of amber”, and leaving a black residue which is known as “amber colophony”, or “amber pitch”; when dissolved in oil ofturpentine or in linseed oil this forms “amber varnish” or “amber lac”.
Amber from the Baltic Sea has been extensively traded since antiquity and in the main land, from where amber was traded 2000 years ago, the natives called it glaes (referring to its see-through similarity to glass).
The Baltic Lithuanian term for amber is Gintaras and Latvian Dzintars. They and the Slavicjantar are thought to originate fromPhoenician jainitar (sea-resin). However, while most Slavic languages, such as Russian and Czech, retain the old Slavic word, in the Polish language, despite still correct, it is used very rarely (even considered archaic) and was replaced by the word bursztyn deriving from the German analogue.

A mosquito and a fly in thisBaltic amber necklace are between 40 and 60 million years old
Chemistry of amber
Amber is heterogeneous in composition, but consists of several resinous bodies more or less soluble in alcohol, ether andchloroform, associated with an insoluble bituminous substance. Amber is a macromolecule by free radical polymerization of several precursors in the labdanefamily, communic acid, cummunol and biformene.[4] These labdanes are diterpenes (C20H32) and trienes which means that the organic skeleton has three alkene groups available forpolymerization. As amber matures over the years, more polymerization will take place as well as isomerization reactions, crosslinking and cyclization. The average composition of amber leads to the general formula C10H16O.
Amber should be distinguished from copal. Molecular polymerisation caused by pressure and heat transforms the resin first into copal and then over time through the evaporation of turpenes it is transformed into amber.
Baltic amber is distinguished from the various other ambers from around the world, by the presence within it of succinic acid,[citation needed] hence Baltic amber is otherwise known as succinite.
Amber in geology
The oldest amber originates from the Upper Carboniferous period approximately 345 million years ago. The oldest known amber containing insects comes from the Lower Cretaceous, approximately 146 million years ago.
Commercially most important are the deposits of Baltic and Dominican amber.[5]

A bee and a leaf inside amber
Baltic amber or succinite (historically documented as Prussian amber) is found as irregular nodules in a marine glauconitic sand, known as blue earth, occurring in the Lower Oligocenestrata of Samland in Prussia (Latin: Sambia), in historical sources also referred to asGlaesaria. After 1945 this territory around Königsberg was turned into Kaliningrad Oblast,Russia, where it is now systematically mined.[6] It appears, however, to have been partly derived from yet earlier Tertiary deposits (Eocene); and it occurs also as a derivative mineral in later formations, such as the drift. Relics of an abundant flora occur as inclusions trapped within the amber while the resin was yet fresh, suggesting relations with the flora of EasternAsia and the southern part of North America.Heinrich Göppert named the common amber-yielding pine of the Baltic forests Pinites succiniter, but as the wood, according to some authorities, does not seem to differ from that of the existing genus it has been also calledPinus succinifera. It is improbable, however, that the production of amber was limited to a single species; and indeed a large number of conifers belonging to different genera are represented in the amber-flora.
Dominican amber is considered retinite, since it has no succinic acid. There are three main sites in the Dominican Republic: La Cordillera Septentrional, in the north, Bayaguana and Sabana, in the east. In the northern area, the amber-bearing unit is formed of clastic rocks, sandstone accumulated in a deltaic or even deep-water environment. The oldest, and hardest of this amber comes from the mountain region north of Santiago area, from the mines at La Cumbre, La Toca, Palo Quemado, La Bucara, and Los Cacaos mining sites in the Cordillera Septentrional not far from Santiago. Amber in these mountains is tightly embedded in a lignite layer of sandstone.
There is also amber in the south-eastern Bayaguana/Sabana area. It is softer, sometimes brittle and suffers oxidation after being taken from the mines, therefore less expensive. There is also copal found with only an age of 15-17 million years. In the eastern area, the amber is found in a sediment formation of organic-rich laminated sand, sandy clay, intercalated lignite as well as some solated beds of gravel and calcarenite.
Both Baltic and Dominican amber are rich sources of fossils and give much information about life in the ancient forests. [7]
Amber from the Middle Cretaceous is known from Ellsworth County, Kansas. This approximately 100 million year old amber has inclusions of bacteria and amoebae. They are morphologically very close to Leptothrix, and the modern genera Pontigulasia andNebela. Morphological stasis is considered to be confirmed.[8]
Amber inclusions

A spider trapped in amber
The resin contains, in addition to the beautifully preserved plant-structures, remains of insects, spiders, annelids, frogs,[9]crustaceans, marine microfossils[10] and other small organisms which were trapped by the sticky surface and became enveloped while the exudation was fluid. In most cases the organic structure has disappeared, leaving only a cavity, with perhaps a trace ofchitin. Even hair and feathers have occasionally been represented among the enclosures. Fragments of wood frequently occur, with the tissues well-preserved by impregnation with the resin; while leaves, flowers and fruits are occasionally found in marvelous perfection. Sometimes the amber retains the form of drops and stalactites, just as it exuded from the ducts and receptacles of the injured trees. It is thought that, in addition to exuding onto the surface of the tree, amber resin also originally flowed into hollow cavities or cracks within trees, thereby leading to the development of large lumps of amber of irregular form.[11]
The abnormal development of resin has been called succinosis. Impurities are quite often present, especially when the resin dropped on to the ground, so that the material may be useless except for varnish-making, whence the impure amber is called firniss. Enclosures ofpyrites may give a bluish color to amber. The so-called black amber is only a kind of jet. Bony amber owes its cloudy opacity to minute bubbles in the interior of the resin.
Not all amber is translucent, becoming transparent when the surfaces are polished, thus revealing inclusions. The technique of inspecting darkly clouded and even opaque amber for inclusions, through bombarding it with high-energy, high-contrast, high-resolution x-rays, is being developed at the European Synchrotron Radiation Facility.[12] Nearly 360 fossil invertebrates have been discovered from opaque amber found at Charentes, France: primitive wasps, flies, ants and spiders, particularly those measuring just a few millimeters. Three-dimensional images of the trapped organisms are built up through microtomography, showing detail on the scales of micrometres. An enlarged plastic three-dimensional model can be obtained of an organism that has remained embedded in the amber, suggesting alternative means of cataloguing new species trapped in amber.
Amber locations
Baltic amber

Lithuanian girls in the national dress, which includes an amber necklace.
Baltic amber has a very wide distribution, extending over a large part of northern Europe and occurring as far east as the Urals.
Baltic amber yields on dry distillation succinic acid, the proportion varying from about 3% to 8%, and being greatest in the pale opaque or bony varieties. The aromatic and irritating fumes emitted by burning amber are mainly due to this acid. Baltic amber is distinguished by its yield of succinic acid, hence the name succinite proposed by Professor James Dwight Dana, and now commonly used in scientific writings as a specific term for the Prussian amber. Succinite has a hardness between 2 and 3, which is rather greater than that of many other fossil resins. Its specific gravity varies from 1.05 to 1.10. An effective tool for Baltic amber analysis is IR spectroscopy. It enables the distinction between Baltic and non-Baltic amber varieties because of a specific carbonylabsorption and it can also detect the relative age of an amber sample. On the other hand, it has been suggested by scientists that succinic acid is no original component of amber, but a degradation product of abietic acid. (Rottlaender, 1970)
Although amber is found along the shores of a large part of the Baltic Sea and the North Sea, the great amber-producing area for many centuries was the promontory of Sambia or Samland, the coast around Königsberg in Prussia, since 1945 part of Russia. About 90% of the world’s extractable amber is still located in the Kaliningrad Oblast of Russia on the Baltic Sea.[13] Pieces of amber torn from the seafloor are cast up by the waves, and collected at ebb-tide. Sometimes the searchers wade into the sea, furnished with nets at the end of long poles, which they drag in the seaweed containing entangled masses of amber; or they dredge from boats in shallow water and rake up amber from between the boulders. Divers have been employed to collect amber from the deeper waters. Systematic dredging on a large scale was at one time carried on in the Curonian Lagoon by Messrs Stantien and Becker, the great amber merchants of Königsberg. At the present time extensive mining operations are conducted in quest of amber. The pit amberwas formerly dug in open works, but is now also worked by underground galleries. The nodules from the blue earth have to be freed from matrix and divested of their opaque crust, which can be done in revolving barrels containing sand and water. The sea-worn amber has lost its crust, but has often acquired a dull rough surface by rolling in sand.
Since the establishment of the Amber Road, amber known as “Prussian gold” (which is now also referred to as “Lithuanian gold”) has substantially contributed economically and culturally. Amber jewellery and amberware is offered to foreign tourists in mostsouvenir shops as distinctive to Lithuania and its cultural heritage. The seaside town of Palanga has the Palanga Amber Museumdedicated to amber. Amber can also be found in Latvia as well as Denmark,northern Germany, Poland, and, since the takeover of Prussia in 1945, also in Russia.
Dominican amber
Dominican amber differentiates itself from Baltic amber by being mostly transparent and often containing a higher number of fossilinclusions. This has enabled the detailed reconstruction of the ecosystem of a long-vanished tropical forest.[14] Resin from the extinct species Hymenaea protera is the source of Dominican amber and probably of most amber found in the tropics. It is not “succinite” but “retinite“. [15] In contrast to much Baltic amber, Dominican amber found on the world market is natural amber the way it comes from the mines, and has not been enhanced or received any chemical or physical change. The age of Dominican amber is up to 40 million years. [16]
Although all Dominican amber is fluorescent, the rarest Dominican amber is blue amber. It turns blue in natural sunlight and any other partially or wholly ultraviolet light source. In long-wave UV light it has a very strong reflection, almost white. Only about 100 kilos of this fossilized tree is found per year, which makes it valuable and expensive.[17]

Dragon carved from Dominican blue amber
Dominican amber, and especially Dominican blue amber, is mined through bell pitting, which is highly dangerous for workers due to the risk of the excavation walls collapsing on them. [18]Bell pitting is basically a foxhole dug with whatever tools are available. Machetes do the start, some shovels, picks and hammers may participate eventually. The pit itself goes as deep as possible or safe, sometimes vertical, sometimes horizontal, but never level. It snakes into hill sides, drops away, joins up with others, goes straight up and pops out elsewhere. Rarely are the pits large enough to stand in, and then only at the entrance. Miners crawl around on their knees using short-handled picks, shovels and machetes. The amber that is found is either directly sold as rough or raw pieces or cut and polished without any additional treatments or enhancements.[14]
The most common use for Dominican amber is as ornaments and jewellery, while the more valuable enclosures and colorations become priced exhibition pieces both in private and public collections. [19] In the Far East, the rare blue Amber has been masterfully worked into artistic carvings. Others have used blue amber to make jewellery that can be especially attractive for its naturalfluorescence under UV lights. In the Muslim world, Dominican amber and particularly blue amber beads have found their way into another use as Prayer beads andworry beads, since Dominican amber can very easily be worked.[20][21]
Other locations
Amber deposits are found around the world. Some are much older than the well known amber deposits in the Baltic countries and the Dominican Republic, others are much younger. Some amber is considered to be up to 345 million years old (Northumberland USA).
A lesser known source of amber is in the Ukraine, within a marshy forested area on the Volyhn-Polesie border. Due to the shallow depth at which this amber is found it can be extracted with the simplest of tools, and this has led to an economy of amber poaching under cover of the forest. This Ukrainian amber has a wide range of colors, and was used in the restoration of Amber Room in the Empress Catherine’s palace in Saint Petersburg (see below).

Sailboat made entirely from amber in a gift shop
Rolled pieces of amber, usually small but occasionally of very large size, may be picked up on the east coast of England, having probably been washed up from deposits under the North Sea. Cromer is the best-known locality, but it occurs also on other parts of the Norfolk coast, such as Great Yarmouth, as well as Southwold, Aldeburgh and Felixstowe in Suffolk, and as far south as Walton-on-the-Naze in Essex, whilst northwards it is not unknown in Yorkshire. On the other side of the North Sea, amber is found at various localities on the coast of theNetherlands and Denmark. On the shores of the Baltic it occurs not only on the German and Polish coast but in the south of Sweden, in Bornholm and other islands, and in southernFinland. Some of the amber districts of the Baltic and North Sea were known in prehistoric times, and led to early trade with the south of Europe through the Amber Road. Amber was carried to Olbia on the Black Sea, Massilia (today Marseille) on the Mediterranean, and Adriaat the head of the Adriatic; and from these centres it was distributed over the Ancient Greekworld.
Amber is found in Switzerland, Austria and France. Amber from the Swiss Alps is about 55 – 200 million years old, amber from Golling about 225 – 231 million years. The well-known Sicilian Amber (Simetit – copal) is just 10 – 20 million years old.
In Africa, copal is found in the coastal countries of East and West Africa, but especially onMadagascar. This so-called MadagascarAmber is only 1,000 – 10,000 years old and consists of the solidified resin of the amber pine. Nigeria also has amber, which is about 60 million years old.
In Asia amber can be found especially in Burma (former Burma / Myanmar) as Burmit. It is about 50 million years and the Lebanon amber 130 – 135 million years old. Amber of the Australian-oceanic area can be found in New Zealand and Borneo (Sarawak amber). They are about 20 – 60, part 70 – 100 million years old.

Rare polished transparent Borneo amber from Sabah, Malaysia
Amber is also found to a limited extent in the United States, as in the green-sand of New Jersey, but it has little economic value. Middle Cretaceous amber has also been found inEllsworth County, Kansas. It has little value for jewelry makers, but is very valuable to biologists. The source of this amber is under a man-made lake.
A fluorescent amber occurs also in the southern state of Chiapas in Mexico, and is used for eye-catching jewellery. In Central America, the Olmec civilization was mining amber around 3000 B.C. There are legends in Mexico that mention the use of amber in adorning, consuming and using it for stress reduction as a natural remedy.
Indonesia is also a rich source of amber with large fragments being unearthed in both Java and Bali.
Amber treatments
The Vienna amber factories which use pale amber to manufacture pipes and other smoking tools, turn it on a lathe and polish it with whitening and water or with rotten stone and oil. The final lustre is given by friction with flannel.
When gradually heated in an oil-bath, amber becomes soft and flexible. Two pieces of amber may be united by smearing the surfaces with linseed oil, heating them, and then pressing them together while hot. Cloudy amber may be clarified in an oil-bath, as the oil fills the numerous pores to which the turbidity is due. Small fragments, formerly thrown away or used only for varnish, are now used on a large scale in the formation of “amberoid” or “pressed amber”. The pieces are carefully heated with exclusion of air and then compressed into a uniform mass by intense hydraulic pressure; the softened amber being forced through holes in a metal plate. The product is extensively used for the production of cheap jewellery and articles for smoking. This pressed amber yields brilliant interference colors in polarized light. Amber has often been imitated by other resins like copal and kauri, as well as by celluloid and even glass. Baltic amber is sometimes colored artificially, but also called “true amber”.
Often amber (particularly with insect inclusions) is counterfeited using a plastic resin. A simple test consists of touching the object with a heated pin and determining if the resultant odor is of wood resin. If not, the object is counterfeit, although a positive test may not be conclusive owing to a thin coat of real resin. Often counterfeits will have a too perfect pose and position of the trapped insect.
Amber art and ornament
Amber was much valued as an ornamental material in very early times. It has been found inMycenaean tombs; it is known from lake-dwellings in Switzerland, and it occurs with Neolithicremains in Denmark, whilst in England it is found with interments of the bronze age.
The so-called Hove amber cup, a cup turned in amber from a bronze-age barrow at Hove is now in the Brighton Museum.
Beads of amber are found with Anglo-Saxon relics in the south of England. Amber was valued as an amulet and it is still believed to possess medicinal properties.
Amber is used for beads and ornaments, and for cigar-holders and the mouth-pieces of pipes. It is regarded by the Turks as specially valuable, inasmuch as it is said to be incapable of transmitting infection as the pipe passes from mouth to mouth. The variety most valued in the East is the pale straw-colored, slightly cloudy amber. Some of the best qualities are sent toVienna for the manufacture of smoking appliances.
The Amber Room was a collection of chamber wall panels commissioned in 1701 for the king of Prussia, then given to Tsar Peter the Great. The room was hidden in place from invadingNazi forces in 1941, who upon finding it in the Catherine Palace, disassembled it and moved it to Königsberg. What happened to the room beyond this point is unclear, but it may have been destroyed when the Russians burned the German fortification where it was stored. It is presumed lost. It was re-created in 2003.[22]
Amber Frog Violin Bow

The Amber Frog bow by Keith Peck made in 1996/97 commissioned by Gennady Filimonov.
Baltic amber has been used to create the “frog” part of a Violin bow. It was commissioned by Gennady Filimonov and made by the late American Master Bowmaker Keith Peck [23]
The Amber Frog / Picture bow (copy of F.N. Voirin), is the first documented amber frog bow (made in 1996-97) , that was (and is) a complete success. It is still being played by Gennady Filimonov |
Rhodochrosite

Pink is the most common color of Rhodochrosite. Specimen mined near Silverton, Colorado
Rhodochrosite is a manganese carbonate mineral with chemical composition MnCO3. In its (rare) pure form, it is typically a rose-red color, but impure specimens can be shades of pink to pale brown. The streak is white. Its Mohs hardness varies between 3.5 and 4. Its specific gravity is 3.5 to 3.7. It crystallizes in the trigonal system. The cleavage is typical rhombohedral carbonate cleavage in three directions. Crystal twinning often is present. It is transparent to translucent with refractive indices of nω=1.814 to 1.816,nε=1.596 to 1.598. It is often confused with the manganese silicate, rhodonite, but is distinctly softer.
Rhodochrosite forms a complete solid solution series with iron carbonate (siderite). Calcium, (as well as magnesium and zinc, to a limited extent) frequently substitutes for manganese in the structure, leading to lighter shades of red and pink, depending on the degree of substitution. It is for this reason that the most common color encountered is pink.
Rhodochrosite occurs as a hydrothermal vein mineral along with other manganese minerals in low temperature ore deposits as in the silver mines of Romania where it was first found. Banded rhodochrosite is mined in Capillitas, Argentina. Catamarca, Argentina has an old Incan silver mine that has produced fine stalactitic examples of rhodochrosite that are unique and very attractive. Cut cross-sections reveal concentric bands of light and dark rose colored layers.. These specimens are carved and used for many ornamental purposes.[3]
Its main use is as an ore of manganese which is a key component of low-cost stainless steel formulations and certain alluminium alloys. Quality banded specimens are often used for decorative stones and jewelry. Due to its being relatively soft, and having perfect cleaveage, it is very difficult to cut, and therefore rarely found faceted in jewelry.
It was first described in 1813 in reference to a sample from Cavnic, Maramureş, present-dayRomania. According to Dimitrescu and Radulescu, 1966 and to Papp, 1997, this mineral was described for the first time in Sacaramb, Romania, not in Cavnic, Romania. The name is derived from the Greek word for rose-colored.
Colorado officially named rhodochrosite as its state mineral in 2002 based on a proposal by a local high school (Platte Canyon High School in Bailey,Colorado). The reason for this lies in the fact that while the mineral is found worldwide, large red crystals are found only in a few places on earth, and some of the best specimens have been found in the Sweet Home Mine near Alma, Colorado.
The Incas believed that rhodochrosite is the blood of their former rulers, turned to stone, therefore it is sometimes called “Rosa del Inca” or “Inca Rose”.[4][5]
Rhodochrosite and silver mining
Manganese carbonate is extremely destructive to the amalgamation process used in the concentration of silver ores, and so until quality mineral specimens became highly sought after by collectors, they
Labradorite
Labradorite ((Ca,Na)(Al,Si)4O8), a feldspar mineral, is an intermediate to calcic member of the plagioclase series. It is usually defined as having “%An” (anorthite) between 50 and 70. The specific gravity ranges from 2.71 to 2.74. The streak is white, like most silicates. Therefractive index ranges from 1.555 to 1.575. Twinning is common. As with all plagioclase members the crystal system is triclinic and three directions of cleavage are present two of which form nearly right angle prisms. It occurs as clear, white to gray, blocky to lath shaped grains in common mafic igneous rocks such as basalt and gabbro, as well as in anorthosites.
The geological type area for labradorite is Paul’s Island near the town of Nain in Labrador,Canada. It occurs in large crystal masses in anorthosite and shows an iridescence or play of colors. The iridescence is the result of light refracting within lamellar intergrowths resulting from phase exsolution on cooling in the Boggild miscibility gap, An48-An58.
Gemstone varieties of labradorite exhibiting a high degree of iridescence are called spectrolite;moonstone and sunstone are also commonly used terms, and high-quality samples with good iridescent qualities are desired for jewelry
Feldspar
- This article is about a mineral. For the Malcolm in the Middle character, see List of characters in Malcolm in the Middle#Recurring characters.
Feldspars (KAlSi3O8 - NaAlSi3O8 - CaAl2Si2O8) are a group of rock-forming tectosilicateminerals which make up as much as 60% of the Earth‘s crust.[1]
Feldspars crystallize from magma in both intrusive and extrusive igneous rocks, as veins, and are also present in many types ofmetamorphic rock.[2] Rock formed entirely of plagioclasefeldspar (see below) is known as anorthosite.[3] Feldspars are also found in many types ofsedimentary rock.[4]
Etymology
Feldspar is derived from the German Feld, field, and Spat, a rock that does not contain ore. “Feldspathic” refers to materials that contain feldspar. The alternative spelling, felspar, has now largely fallen out of use.[5]
Compositions

Compositional phase diagram of the different minerals that constitute the feldspar solid solution.

Alkali feldspar perthite (7cm long X 3cm width).
This group of minerals consists of framework or tectosilicates. Compositions of major elements in common feldspars can be expressed in terms of three endmembers:
Potassium-Feldspar (K-spar) endmember KAlSi3O8[1]
Albite endmember NaAlSi3O8[1]
Anorthite endmember CaAl2Si2O8[1]
Solid solutions between K-feldspar and albite are called alkali feldspar.[1] Solid solutions between albite and anorthite are calledplagioclase,[1] or more properly plagioclase feldspar. Only limited solid solution occurs between K-feldspar and anorthite, and in the two other solid solutions, immiscibility occurs at temperatures common in the crust of the earth. Albite is considered both a plagioclase and alkali feldspar. In addition to albite, barium feldspars are also considered both alkali and plagioclase feldspars. Barium feldspars form as the result of the replacement of potassium feldspar.
Alkali Feldspars
The alkali feldspars are as follows:
Sanidine is stable at the highest temperatures, and microcline at the lowest.[7][6] Perthite is a typical texture in alkali feldspar, due toexsolution of contrasting alkali feldspar compositions during cooling of an intermediate composition. The perthitic textures in the alkali feldspars of many granites can be seen with the naked eye.[9] Microperthitic textures in crystals are visible using a light microscope, whereas cryptoperthitic textures can only be seen using an electron microscope.
Plagioclase Feldspars
The plagioclase feldspars are triclinic. The plagioclase series follows (with percent anorthite in parentheses):
Intermediate compositions of plagioclase feldspar also may exsolve to two feldspars of contrasting composition during cooling, but diffusion is much slower than in alkali feldspar, and the resulting two-feldspar intergrowths typically are too fine-grained to be visible with optical microscopes. The immiscibility gaps in the plagioclase solid solution are complex compared to the gap in the alkali feldspars. The play of colors visible in some feldspar of labradorite composition is due to very fine-grained exsolution lamellae.
Barium Feldspars
The barium feldspars are monoclinic and comprise the following:
Feldspars can form clay minerals through chemical weathering..[10]
Uses

Feldspar output in 2005. Click the image for the details.
In 2005, Italy was the top producer of feldspar with almost one-fifth world share followed by Turkey, China and Thailand, reports theInternational Monetary Fund.
Hematite

Hematite in SEM, magnification 100x
Hematite, also spelled as hæmatite, is the mineral form of Iron(III) oxide (Fe2O3), one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the samecrystal structure as ilmenite and corundum. Hematite and ilmenite form a completesolid solution at temperatures above 950°C.
Hematite is a mineral, colored black to steel or silver-gray, brown to reddish brown, or red. It ismined as the main ore of iron. Varieties include kidney ore, martite (pseudomorphs aftermagnetite), iron rose and specularite (specular hematite). While the forms of hematite vary, they all have a rust-red streak. Hematite is harder than pure iron, but much more brittle.Maghemite is a hematite- and magnetite-related oxide mineral.
Huge deposits of hematite are found in banded iron formations. Grey hematite is typically found in places where there has been standing water or mineral hot springs, such as those inYellowstone National Park in the United States. The mineral can precipitateout of water and collect in layers at the bottom of a lake, spring, or other standing water.. Hematite can also occur without water, however, usually as the result of volcanic activity.
Clay-sized hematite crystals can also occur as a secondary mineral formed by weatheringprocesses in soil, and along with other iron oxides or oxyhydroxides such as goethite, is responsible for the red color of many tropical, ancient, or otherwise highly weathered soils.
Good specimens of hematite come from England, Mexico, Brazil, Australia, United States andCanada.
Etymology and history

Ancient Egyptian cylindrical seal (left) made from hematite with corresponding impression (right), approximately 14th century BC
The name hematite is derived from the Greek word for blood (haima ἁιμα) because hematite can be red, as in rouge, a powdered form of hematite. The color of hematite lends it well in use as a pigment.
Ochre is a clay that is colored by varying amounts of hematite, varying between 20% and 70%[3]. Red ochre contains unhydrated hematite, whereas yellow ochre contains hydratedhematite (Fe2O3 • H2O).. The principal use of ochre is for tinting with a permanent color[3].
The red chalk winning of this mineral was one of the earliest in history of mankind. The powdery mineral was first used 164,000 years ago by the Pinnacle-Point man obviously for social differentiation[4]. Hematite residues are also found in old graveyards from 80,000 years ago. Near Rydno in Poland and Lovas in Hungary, palaeolitic red chalk mines have been found that are from 5000 BC, belonging to the Linear Pottery culture at the Upper Rhine.
Rich deposits of hematite have been found on the island of Elba that have been mined till the time of the Etruscans.
Ancient Egyptian booby trap
In 2001, Egyptian government archaeologist Zahi Hawass was the first to enter a previously undisturbed tomb, believed to be that of an ancient regional mayor, in the Bahariya Oasisbelow the town of Bawiti. Upon entering the burial chamber, Hawass discovered abooby trapconsisting of 8 inches of finely powdered hematite dust covering the floor and sarcophagus.[5]When disturbed by a tomb robber, the sharp, metallic dust was intended to become airborne and irritate the skin, eyes and mucous membranes, eventually causing lethal siderosis if exposed for long enough. The archaeological team was forced to retreat and don full body suits and respirators in order to confirm the identity of the mummy. Hawass cites the ancient Egyptians’ experience with powdered hematite as a paint pigment as proof that they were aware of its irritating properties.[6]
Jewelry

Hematite carving, 5 cm (2 in) long.
Hematite’s popularity in jewelry was at its highest in Europe during the Victorian era, and has since seen a strong resurgence inNorth America, especially in the western United States.. Due to it delicate nature, the mineral is found only in precious jewelry. Extreme care should be taken in handling hematite items due to the material’s susceptibility to irreversible damage.
It is also used in art such as intaglios where it is used for making hollow portraits.
Magnetism

crystal structure of hematite
Hematite is an antiferromagnetic material below the Morin transition at 250 K, and a canted antiferromagnet or weakly ferromagneticabove the Morin transition and below its Néel temperature at 948K, above which it is paramagnetic.
The magnetic structure of a-hematite was the subject of considerable discussion and debate in the 1950s because it appeared to be ferromagnetic with a Curie temperature of around 1000 K, but with an extremely tiny moment (0.002 µB). Adding to the surprise was a transition with a decrease in temperature at around 260 K to a phase with no net magnetic moment. It was shown that the system is essentially antiferromagnetic but that the low symmetry of the cation sites allows spin–orbit coupling to cause canting of the moments when they are in the plane perpendicular to the c axis. The disappearance of the moment with a decrease in temperature at 260 K is caused by a change in the anisotropy which causes the moments to align along the c axis. In this configuration, spin canting does not reduce the energy.[7][8]
Hematite is part of a complex solid solution oxyhydroxide system having various degrees of water, hydroxyl group, and vacancy substitutions that affect the mineral’s magnetic and crystal chemical properties.[9] Two other end-members are referred to as protohematite and hydrohematite.
Iron from mine tailings
Hematite is present in the waste tailings of iron mines. A recently developed process,magnetation, uses huge magnets to glean waste hematite from old mine tailings inMinnesota‘s vast Mesabi Range iron district.[10]
Hematite on Mars

Image mosaic from the Mars Exploration Rover Microscopic Imager shows Hematitespherules partly embedded in rock at the Opportunity landing site. (Scale: image is approximately 5 cm (2 in) across)
The spectral signature of hematite was seen on the planet Mars by the infrared spectrometeron the NASA Mars Global Surveyor(“MGS”) and 2001 Mars Odyssey spacecraft in orbit around Mars.[11] The mineral was seen in abundance at two sites [12] on the planet, the Terra Meridiani site, near the Martian equator at 0° longitude, and the second site Aram Chaos near the Valles Marineris.[13] Several other sites also showed hematite, e.g., Aureum Chaos.[14]Because terrestrial hematite is typically a mineral formed in aqueous environments, or by aqueous alteration, this detection was scientifically interesting enough that the second of the two Mars Exploration Rovers was targeted to a site in the Terra Meridiani region designatedMeridiani Planum. In-situ investigations by the Opportunity rover showed a significant amount of hematite, much of it in the form of small spherules that were informally tagged by the science team “blueberries”. Analysis indicates that these spherules are apparentlyconcretions formed from a water solution.
Zoisite
- This article is about the mineral named zoisite. For the Sailor Moon character, see Shitennou.
| Zoisite |

Anyolite (left) & tanzanite |
| General |
| Category |
Sorosilicate – epidote group |
| Chemical formula |
Ca2Al3(SiO4)(Si2O7)O(OH) |
| Strunz classification |
VIII/C.23-100 |
| Dana classification |
58.2.1b.1 |
| Identification |
| Color |
White, gray, greenish brown, greenish gray, pink, blue, purple |
| Crystal habit |
Crystals flattened in an acicular manner, may be fibrously curved and striated. Massive to columnar |
| Crystal system |
Orthorhombic – Dipyramidal |
| Cleavage |
Perfect {010} imperfect {100} |
| Fracture |
Uneven to conchoidal |
| Mohs Scalehardness |
6 to 7 |
| Luster |
Vitreous, pearly on cleavage surfaces |
| Streak |
White or colorless |
| Diaphaneity |
Transparent to translucent |
| Specific gravity |
3.10-3.36 |
| Optical properties |
biaxial positive |
| Refractive index |
nα = 1..696 – 1.700 nβ = 1.696 – 1.702 nγ = 1.702 – 1.718 |
| Birefringence |
0.006-0.018 |
| Pleochroism |
X = pale pink to red-violet; Y = nearly colorless to bright pink or deep blue; Z = pale yellow to yellow-green |
| References |
[1][2][3] |
| Major varieties |
| Tanzanite |
Gem-quality zoisite, blue-purple |
| Thulite |
Pink |
Zoisite is a calcium aluminium hydroxy sorosilicate belonging to the epidote group ofminerals. Its chemical formula isCa2Al3(SiO4)(Si2O7)O(OH). Zoisite is named after theSlovene scientist Baron Sigmund Zois von Edelstein (Žiga Zois), who realized that this was an unknown mineral when it was brought to him by the mineral dealer Simon Prešern, who had discovered it in theSaualpe mountains (Svinška planina) of Carinthia in 1805. Zoisite was first known as saualpite, after its type locality.
Zoisite occurs as prismatic, orthorhombic (2/m 2/m 2/m) crystals or in massive form, being found in metamorphic and pegmatiticrock. Zoisite may be blue to violet, green, brown, pink, yellow, gray, or colorless. It has a vitreous luster and a conchoidal to unevenfracture. When euhedral, zoisite crystals are striated parallel to the principal axis (c-axis). Also parallel to the principal axis is one direction of perfect cleavage. Zoisite is somewhat higher than 6 inhardness and its specific gravity is between 3.10 – 3.38, depending on the variety. Zoisite streaks white and is said to be brittle. Clinozoisite is a more common monoclinic polymorph of zoisite.
Transparent material is fashioned into gemstones while translucent-to-opaque material is usually carved. A metamorphic rock known as anyolite consists of green zoisite with blacktschermakite and ruby crystals.[4][5]
Sources of zoisite include Tanzania (tanzanite), Kenya (anyolite), Norway (thulite),Switzerland, Austria, India, Pakistan, andWashington in the USA. |
Nephrite
| Nephrite |

Nephrite |
| General |
| Category |
Mineral |
| Chemical formula |
Ca2(Mg,Fe)5Si8O22(OH)2[1] |
| Identification |
| Color |
Translucent to opaque and often mottled. Light to dark green, yellow to brown, white, gray, black.[1] |
| Crystal habit |
massive[1] |
| Crystal system |
monoclinic[1] |
| Fracture |
splintery to granular[1] |
| Mohs Scalehardness |
6 – 6.5[1] |
| Luster |
dull[1] |
| Specific gravity |
2.95 (+.15, -.05)[1] |
| Polish luster |
vitreous to greasy[1] |
| Optical properties |
Double refractive with anomalous aggregate reaction[1] |
| Refractive index |
1.606 – 1.632 (+.009, -.006)[1] |
| Birefringence |
usually not detectable[1] |
| Pleochroism |
none[1] |
| Ultravioletfluorescence |
inert[1] |
| Absorption spectra |
Vague line may be present at 500 nm, but rarely any lines. Rarely, in stones of exceptional gem quality, vague lines in the red part of the spectrum may be seen.[1] |
Nephrite is a variety of the calcium and magnesium-rich amphibole mineral actinolite(aggregates of which also make up one form ofasbestos). The chemical formula for nephrite isCa2(Mg,Fe)5Si8O22(OH)2.[1] It is one of two different mineral species called jade. The other mineral species known as jade is jadeite, which is a variety of pyroxene. While nephrite jade possess mainly grays and greens (and occasionally yellows, browns or whites), Jadeite jade, which is rarer, can also contain blacks, reds, pinks and violets. Nephrite jade is an ornamental stone, used in carvings, beads, or cabochon cut gemstones.
The name nephrite is derived from lapis nephriticus, which means ‘kidney stone’ and is the Latin version of the Spanish piedra de ijada.[2] Accordingly, nephrite jade was once believed to be a cure for kidney stones.
Nephrite can be found in a translucent white to very light yellow form which is known in China as mutton fat jade,[1] in an opaque white to very light brown or gray which is known aschicken bone jade,[1] as well as in a variety of green colours. Canada is the principal source of modern lapidary nephrite. Nephrite jade was used mostly in pre-1800 China as well as in New Zealand, the Pacific Coast and Atlantic Coasts of North America, Neolithic Europe, and southeast Asia.
History
Prehistoric and historic China
During Neolithic times, the key known sources of nephrite jade in China for utilitarian and ceremonial jade items were the now depleted deposits in the Ningshao area in the Yangtze River Delta (Liangzhu culture 3400–2250 BC) and in an area of the Liaoning province in Inner Mongolia (Hongshan culture 4700–2200 BC). Jade was used to create many utilitarian and ceremonial objects, ranging from indoor decorative items to jade burial suits. Jade was considered the “imperial gem”. From about the earliest Chinese dynasties until present, the jade deposits in most use were from the region of Khotan in the Western Chinese province ofXinjiang(jade deposits from other areas of China, such as Lantian, Shaanxi, were also in great demand). There, white and greenish nephrite jade is found in small quarries and as pebbles and boulders in the rivers flowing from the Kuen-Lun mountain range northward into theTakla-Makan desert area. River jade collection was concentrated in the Yarkand, the White Jade (Yurungkash) and Black Jade (Karakash) Rivers. From the Kingdom of Khotan, on the southern leg of the Silk Road, yearly tribute payments consisting of the most precious white jade were made to the Chinese imperial court and there transformed into objets d’art by skilled artisans, as jade was considered more valuable than gold or silver.
Māori
Nephrite jade in New Zealand is known as pounamu in the Māori language, and is highly valued, playing an important role in Māoriculture. It is considered a taonga, or treasure, and therefore protected under the Treaty of Waitangi, and the exploitation of it is restricted and closely monitored. The South Island of New Zealand is Te Wai Pounamu in Māori — “The [land of] Greenstone Water” — because that is where it occurs.
Weapons and ornaments were made of it; in particular the mere (short club), and the hei-tiki(neck pendant). These were believed to have their own mana, were handed down as valuable heirlooms, and often given as gifts to seal important agreements. It was also used for a range of tools such as adzes, as Māori had no metal tools.
In New Zealand English its normal name is “greenstone”. Jade jewellery in Māori designs is widely popular with locals of all races, and with tourists – although much of the jade itself is now imported from British Columbia and elsewhere.
Other names
Besides the terms already mentioned, nephrite has the following synonyms and varieties:aotea, axe-stone, B.C. jade, beilstein,kidney stone, lapis nephriticus, nephrit, nephrita, New Zealand greenstone,[1] New Zealand jade,[1] spinach jade (dark grayish green),[1] and talcum nephriticus. Tomb jade or grave jade are names given to ancient burial nephrite pieces that have a brown or chalky white texture as a surface treatment.[1]
Jade
“Nephrite jade” redirects here. You may be looking for Nephrite.

A selection of antique, hand craftedChinesejade (jadeite) buttons.
Jade is an ornamental stone. The term jade is applied to two different metamorphic rocks that are made up of different silicate minerals:
- Nephrite jade, consists of a microcrystaline interlocking fibrous matrix of the calcium, magnesium-iron rich amphibole mineral series tremolite (calcium-magnesium)-ferroactinolite (calcium-magnesium-iron). The middle member of this series with an intermediate composition is calledactinolite (the silky fibrous mineral form is one form of asbestos). The higher the iron content the greener the colour.
- Jadeitite, a rock consisting almost entirely of jadeite, a sodium- and aluminium-rich pyroxene. The gem form of the mineral is a microcrystaline interlocking crystal matrix.
The English word jade is derived from the Spanish term piedra de ijada (first recorded in 1565) or “loin stone”, from its reputed efficacy in curing ailments of the loins and kidneys. Nephrite is derived from lapis nephriticus, the Latin version of the Spanish piedra de ijada.[1]
Nephrite and jadeite were used by people from the prehistoric for similar purposes. Jadeite has about the same hardness as quartz, while nephrite is somewhat softer. Both nephrite and jadeite are tough, but nephrite is tougher than jadeite. They can be delicately shaped. Thus it was not until the 19th century that a French mineralogist determined that “jade” was in fact two different materials. The trade name jadite is sometimes applied to translucent or opaque green glass.
Among the earliest known jade artifacts excavated from prehistoric sites are simple ornaments with bead, button, and tubular shapes.[2] Additionally, jade was used for axe heads, knives, and other weapons. As metal-working technologies became available, the beauty of jade made it valuable for ornaments and decorative objects. Jadeite measures between 6.5 and 7.0 Mohs hardness, and Nephrite between 5.5 and 6.0,[3] so it can be worked with quartz or garnet sand, and polished with bamboo or even ground jade.
Nephrite can be found in a creamy white form (known in China as “mutton fat” jade) as well as in a variety of green colours, whereas jadeite shows more colour variations, including blue, lavender-mauve, pink, and emerald-green colours. Of the two, jadeite is rarer, documented in fewer than 12 places worldwide. Translucent emerald-green jadeite is the most prized variety, both today and historically. As “quetzal” jade, bright green jadeitite from Guatemala was treasured by Mesoamerican cultures, and as “kingfisher” jade, vivid green rocks from Burma became the preferred stone of post-1800 Chinese imperial scholars and rulers. Burma (Myanmar) and Guatemala are the principal sources of modern gem jadeitite, and Canada of modern lapidary nephrite. Nephrite jade was used mostly in pre-1800 China as well as in New Zealand, the Pacific Coast and Atlantic Coasts of North America, Neolithic Europe, and south-east Asia.. In addition to Mesoamerica, jadeite was used by Neolithic Japanese and European cultures.
Jade is the official gemstone of British Columbia, where it is found in large deposits in the Lillooet andCassiar regions. It is also the official gemstone of the state of Alaska, found particularly in the Kobuk area. A two-ton block of jade sits outside the Anchorage Visitor’s Center in downtown Anchorage, Alaska, mined from near Kobuk and donated to the city as a showpiece. Jade is also the state gemstone of the State ofWyoming.[citations needed]
The 2008 Summer Olympic medals have a ring of jade in them.
History
Prehistoric and Historic China
During Neolithic times, the key known sources of nephrite jade in China for utilitarian and ceremonial jade items were the now depleted deposits in the Ningshao area in the Yangtze River Delta (Liangzhu culture3400–2250 BC) and in an area of the Liaoning province and Inner Mongolia (Hongshan culture 4700–2200 BC).[4] As early as 6000 BC Dushan Jade has been mined.. In the Yin Ruins of Shang Dynasty (1600 BC to 1050 BC) in Anyang, Dushan Jade ornaments was unearthed in the tomb of the Shang kings. Jade was used to create many utilitarian and ceremonial objects, ranging from indoor decorative items to jade burial suits. Jade was considered the “imperial gem”. From about the earliest Chinese dynasties until present, the jade deposits in most use were not only from the region of Khotan in the Western Chinese province ofXinjiang but also from other parts of China, such as Lantian, Shaanxi. There, white and greenish nephrite jade is found in small quarries and as pebbles and boulders in the rivers flowing from the Kuen-Lun mountain range northward into the Takla-Makan desert area. River jade collection was concentrated in theYarkand, the White Jade (Yurungkash) and Black Jade (Karakash) Rivers. From the Kingdom of Khotan, on the southern leg of the Silk Road, yearly tribute payments consisting of the most precious white jade were made to the Chinese Imperial court and there transformed intoobjets d’art by skilled artisans as jade was considered more valuable than gold or silver. Jade became a favorite material for the crafting of Chinese scholars objects, such as rests for calligraphy brushes, as well as the mouthpieces of some opium pipes, due to the belief that breathing through jade would bestow longevity upon smokers who used such a pipe.[5]
Jadeite, with its bright emerald-green, pink, lavender, orange and brown colours was imported from Burmato China only after about 1800. The vivid green variety became known as Feicui (翡翠) or Kingfisher (feathers) Jade. It quickly replaced nephrite as the imperial variety of jade.
In the long history of the art and culture of the enormous Chinese empire, jade has always had a very special significance, roughly comparable with that of gold and diamonds in the West. Jade was used not only for the finest objects and cult figures, but also in grave furnishings for high-ranking members of the imperial family.
Prehistoric and Early Historic Korea
The use of jade and other greenstone was a long-term tradition in Korea (c. 850 BC – AD 668). Jade is found in small numbers of pit-houses and burials. The craft production of small comma-shaped and tubular ‘jades’ using materials such as jade, microcline, jasper, etc in southern Korea originates from the MiddleMumun Pottery Period (c. 850–550 BC).[6] Comma-shaped jades are found on some of the gold crowns ofSilla royalty (c. AD 300/400–668) and sumptuous elite burials of the Korean Three Kingdoms. After the state of Silla united the Korean Peninsula in AD 668, the widespread popularisation of death rituals related to Buddhism resulted in the decline of the use of jade in burials as prestige mortuary goods.
Māori
Nephrite jade in New Zealand is known as pounamu in the Māori language, playing an important role inMāori culture. It is considered a taonga, or treasure, and therefore protected under the Treaty of Waitangi, and the exploitation of it is restricted and closely monitored. It is found only in the South Island of New Zealand, known as Te Wai Pounamu in Māori — “The [land of] Greenstone Water”, or Te Wahi Pounamu— “The Place of Greenstone”.
Tools, weapons and ornaments were made of it; in particular adzes, the ‘mere‘ (short club), and the Hei-tiki(neck pendant). These were believed to have their own mana, handed down as valuable heirlooms, and often given as gifts to seal important agreements.
One name used for nephrite jade in New Zealand English is “greenstone.” While widely used to describe the material used for jewellery items made for the tourist trade, it is a misnomer and simply engenders confusion. The stone should be correctly referred to as “nephrite” or “nephrite jade”. Nephrite jewellery of Maori design is widely popular with locals and tourists, although some of the jade used for these is now imported from British Columbia and elsewhere.[7]
Mesoamerica

Jadeite Pectoral from theMayan Classic period. (195 mm/7.7 in high)
|
|
|
Jade was a rare and valued material in pre-Columbian Mesoamerica. The only source from which the various indigenous cultures, such as theOlmec and Maya, for example, could obtain jade was located in theMotagua River valley in Guatemala. Jade was largely an elite good, and was usually carved in a variety ways, whether serving as a medium upon which hieroglyphs were inscribed, or shaped into symbolic figurines.. Generally, the material was highly symbolic, and it was often employed in the performance of ideological practices and rituals.
Today, Guatemala produces jadeite in a variety of colours, ranging from soft translucent lilac, blue, green, yellow, and black. It is also the source of new colours, including “rainbow jade” and the unique “Galactic Gold,” a black jadeite with natural incrustations of gold, silver and platinum.[8]
Prehistoric and Historic India
The Jainist temple of Kolanpak in the Nalgonda district, Andhra Pradesh,India is home to a 5-foot (1.5 m) high sculpture of Mahavira that is carved entirely out of jade. The is the largest sculpture made from a single jade rock in the world.
Other names
Besides the terms already mentioned, jadeite and nephrite are sometimes referred to by the following:
Jadeite
- Agate verdâtre
- Feitsui
- Jadeit
- Jadeita
- Natronjadeit
- Yunnan Jade
- Yu-stone
Nephrite
- Aotea
- Axe-stone
- B.C. Jade
- Beilstein
- British Columbian Jade
- Canadian Jade
- Dushan Jade
- Nanyang Jade
- Du Jade
- Henan Yu
- Grave Jade
- Kidney Stone
- Lapis Nephriticus
- Nephrit
- Nephrita
- Nephrite (of Werner)
- New Zealand Greenstone
- New Zealand Jade
- Siberian Jade
- Sinkiang jade
- Spinach Jade
- Talcum Nephriticus
- Tomb Jade
Faux jade
Many minerals are sold as jade. Some of these are: serpentine (also bowenite), carnelian, aventurine quartz, glass, grossularite,Vesuvianite, soapstone (and other steatites such as shoushan stone) and recently, Australian chrysoprase. “Korean jade,” “Suzhou jade,” “Styrian jade,” “Olive jade”, and “New jade” are all really serpentine; “Transvaal jade” or “African jade” is grossularite; “Peace jade” is a mixture of serpentine, stichtite, and quartz; “Mountain jade” is dyed dolomite marble.
In almost all dictionaries, the Chinese character ‘yù’ (玉) is translated into English as ‘jade’. However, this frequently leads to misunderstanding: Chinese, Koreans, and Westerners alike generally fail to appreciate that the cultural concept of ‘jade’ is considerably broader in China and Korea than in the West. A more accurate translation for this character on its own would be ‘precious/ornamental rock’. It is seldom, if ever, used on its own to denote ‘true’ jade in Mandarin Chinese; for example, one would normally refer to ‘ying yu’ (硬玉, ‘hard jade’) for jadeite, or ‘ruan yu’ (軟玉, ‘soft jade’) for nephrite. The Chinese names for many ornamental non-jade rocks also incorporate the character ‘yù’, and it is widely understood by native speakers that such stones are not, in fact, true precious nephrite or jadeite. Even so, for commercial reasons, the names of such stones may well still be translated into English as ‘jade’, and this practice continues to confuse the unwary.
Enhancement
Jade may be enhanced (sometimes called “stabilized”). There are three main methods, sometimes referred to as the ABC Treatment System:
- Type A jadeite has not been treated in any way except surface waxing.
- Type B treatment involves exposing a promising but stained piece of jadeite to chemical bleaches and/or acids and impregnating it with a clear polymer resin. This results in a significant improvement of transparency and colour of the material. Currently, infrared spectroscopy is the most accurate test for the detection of polymer in jadeite.
- Type C jade has been artificially stained or dyed. The red colour of Red jade can be enhanced with heat. The effects are somewhat uncontrollable and may result in a dull brown. In any case, translucency is usually lost.
- B+C jade is a combination of B and C: it has been both artificially dyed AND impregnated.
- Type D jade refers to a composite stone such as a doublet comprising a jade top with a plastic backing.[9]
Gallery of Chinese jades
Agate
Agate (pronounced /ˈæɡət/) is a microcrystalline variety of quartz (silica), chiefly chalcedony, characterised by its fineness of grain and brightness of color. Although agates may be found in various kinds of rock, they are classically associated with volcanic rocks but can be common in certainmetamorphic rocks.[1]
Colorful agates and other chalcedonies were obtained over 3,000 years ago from the Achates River, now called Dirillo, in Sicily.[2]
The stone was given its name by Theophrastus, a Greek philosopher and naturalist, who discovered the stone along the shore line of the river Achates (Greek: Αχάτης) sometime between the 4th and 3rd centuries BC.[3] The agate has been recovered at a number of ancient sites, indicating its widespread use in the ancient world; for example, archaeological recovery at the Knossos site on Creteillustrates its role in Bronze Age Minoanculture.[4]
Formation and characteristics
Most agates occur as nodules in volcanic rocks or ancient lavas where they represent cavities originally produced by the disengagement of volatilesin the molten mass which were then filled, wholly or partially, by siliceous matter deposited in regular layers upon the walls. Such agates, when cut transversely, exhibit a succession of parallel lines, often of extreme tenuity, giving a banded appearance to the section. Such stones are known as banded agate, riband agate and striped agate.
In the formation of an ordinary agate, it is probable that waters containing silica in solution—derived, perhaps, from the decomposition of some of the silicates in the lava itself—percolated through the rock and deposited a siliceous coating on the interior of the vapour-vesicles. Variations in the character of the solution or in the conditions of deposition may cause a corresponding variation in the successive layers, so that bands of chalcedony often alternate with layers of crystalline quartz. Several vapour-vesicles may unite while the rock is still viscous, and thus form a large cavity which may become the home of an agate of exceptional size; thus a Brazilian geode lined with amethyst and weighing 67 tons was exhibited at theDusseldorf Exhibition of 1902. Perhaps the most comprehensive review of agate chemistry is a recent text by Moxon cited below.
The first deposit on the wall of a cavity, forming the “skin” of the agate, is generally a dark greenish mineral substance, likeceladonite, delessite or “green earth“, which are rich in iron probably derived from the decomposition of the augite in the enclosing volcanic rock. This green silicate may give rise by alteration to a brown iron oxide (limonite), producing a rusty appearance on the outside of the agate-nodule. The outer surface of an agate, freed from its matrix, is often pitted and rough, apparently in consequence of the removal of the original coating. The first layer spread over the wall of the cavity has been called the “priming”, and upon this base zeolitic minerals may be deposited.
Many agates are hollow, since deposition has not proceeded far enough to fill the cavity, and in such cases the last deposit commonly consists of quartz, often amethyst, having the apices of the crystals directed towards the free space so as to form a crystal-lined cavity, or geode.
On the disintegration of the matrix in which the agates are embedded, they are set free. The agates are extremely resistant to weathering and remain as nodules in the soil or are deposited as gravel in streams and shorelines.
Types of agate
Banded agate (agate-like onyx). The specimen is 2.5 cm (1 inch) wide.
|
|
|
“Turritella agate” (Elimia tenera) from Green River Formation, Wyoming
|
|
|
A Mexican agate, showing only a single eye, has received the name of cyclops agate. Included matter of a green, golden, red, black or other color or combinations embedded in the chalcedony and disposed in filaments and other forms suggestive of vegetable growth, gives rise to dendritic or moss agate. Dendritic agates have fern like patterns in them formed due to the presence of manganese and iron oxides. Other types of included matter deposited during agate-building include sagenitic growths (radial mineral crystals) and chunks of entrapped detritus (such as sand, ash, or mud). Occasionally agate fills a void left by decomposed vegetative material such as a tree limb or root and is called limb cast agate due to its appearance.
Turritella agate is formed from silicified fossil Turritella shells. Turritella are spiral marine gastropods having elongated, spiral shells composed of many whorls. Similarly, coral, petrified wood and other organic remains or porous rocks can also become agatized. Agatized coral is often referred to as Petoskey stone or agate.
Greek agate is a name given to pale white to tan colored agate found in Sicily back to 400 B.C. The Greeks used it for making jewelry and beads. Today any agate of this color from Sicily, once an ancient Greek colony, is called Greek agate. Yet the stone had been around centuries before that and was known to both the Sumerians and the Egyptians, who used the gem for decoration and religious ceremony.
Another type of agate is Brazilian agate, which is found as sizable geodes of layered nodules. These occur in brownish tones interlayered with white and gray. Quartz forms within these nodules, creating a striking specimen when cut opposite the layered growth axis. It is often dyed in various colors for ornamental purposes.
Certain stones, when examined in thin sections by transmitted light, show a diffraction spectrum due to the extreme delicacy of the successive bands, whence they are termed rainbow agates. Often agate coexists with layers or masses of opal, jasper or crystalline quartz due to ambient variations during the formation process.
Other forms of agate include carnelian agate (usually exhibiting reddish hues), Botswana agate, Ellensburg blue agate, blue lace agate, plume agates, tube agate (with visible flow channels), fortification agate (which exhibit little or no layered structure), fire agate (which seems to glow internally like an opal) and Mexican crazy-lace agate (which exhibits an often brightly colored, complex banded pattern) also called Rodeo Agate and Rosetta Stone depending on who owned the mine at the time.
Uses in industry and art
Industry uses agates chiefly to make ornaments such as pins, brooches, paper knives, inkstands, marbles and seals. Because of its hardness and ability to resist acids, agate is used to make mortars and pestles to crush and mix chemicals. Because of the high polish possible with agate it has been used for centuries for leather burnishing tools. Idar-Oberstein was one of the centers which made use of agate on an industrial scale. Where in the beginning locally found agates were used to make all types of objects for the European market, this became a globalized business around the turn of the 20th century: Idar-Oberstein imported large quantities of agate from Brazil, as ship’s ballast. Making using of a variety of proprietary chemical processes, they produced colored beads that were sold around the globe.[5] Agates have long been used in arts and crafts. The sanctuary of a Presbyterian church in Yachats, Oregon, has six windows with panes made of agates collected from the local beaches.
Chrysoprase
Chrysoprase or chrysophrase is a gemstone variety of chalcedony (a cryptocrystalline form of silica) that contains small quantities of nickel. Its color is normally apple-green, but varies to deep green. The darker varieties of chrysoprase are also referred to asprase. (However, the term prase is also used to describe chlorite-included quartz, and to a certain extent is a color-descriptor, rather than a rigorously defined mineral variety.)
Chrysoprase is cryptocrystalline, which means that it is composed of crystals so fine that they cannot be seen as distinct particles under normal magnification. This sets it apart from rock crystal, amethyst, citrine, and the other varieties of crystalline quartz which are basically transparent and formed from easily recognized six-sided crystals. Other members of the cryptocrystalline silica family include agate, carnelian, and onyx. Unlike many non-transparent silica minerals, it is the color of chrysoprase, rather than any pattern of markings, that makes it desirable. The word chrysoprase comes from the Greek chrysos meaning ‘gold’ and prason, meaning ‘leek’.
Unlike emerald which owes its green color to the presence of chromium, the color of chrysoprase is due to trace amounts of nickelcompounds in form of very small inclusions. The nickel reportedly occurs as different silicates, like kerolite or pimelite (not NiO mineral, bunsenite, as was reported before). Chrysoprase results from the deep weathering orlateritization of nickeliferousserpentinites or other ultramafic ophiolite rocks. In the Australian deposits, chrysoprase occurs as veins and nodules with browngoethite and other iron oxidesin the magnesite-rich saprolite below an iron and silica cap.
As with all forms of chalcedony, chrysoprase has a hardness of 6 – 7 on the Mohs hardness scale and a conchoidal fracture like flint.
The best known sources of chrysoprase are Queensland, Western Australia, Germany,Poland, Russia, Arizona, California, andBrazil. The chrysoprase and Ni silicate ore deposit in Szklary, Lower Silesia, Poland, was probably the biggest European chrysoprase occurrence and possibly also the biggest in the world.
A very similar mineral to chrysoprase is chrome chalcedony, in which the color is provided bychromium rather than nickel.
Chalcedony
Chalcedony is a cryptocrystalline form of silica, composed of very fine intergrowths of the minerals quartz and moganite[2]. These are both silica minerals, but they differ in that quartz has a trigonal crystal structure, whilst moganite is monoclinic.
Chalcedony has a waxy luster, and may be semitransparent or translucent. It can assume a wide range of colors, but those most commonly seen are white to gray, grayish-blue or a shade of brown ranging from pale to nearly black.
Varieties
Chalcedony occurs in a wide range of varieties. Many semi-precious gemstones are in fact forms of chalcedony. The more notable varieties of chalcedony are as follows:
Agate
Agate is a variety of chalcedony with multi-colored concentric banding.
Carnelian
Carnelian (also spelled cornelian) is a clear-to-translucent reddish-brown variety of chalcedony. Its hue may vary from a pale orange, to an intense almost-black coloration. Similar to carnelian is sard, which is brown rather than red.
Chrysoprase
Chrysoprase (also spelled chrysophrase) is a green variety of chalcedony, which has been colored by nickel oxide. (The darker varieties of chrysoprase are also referred to as prase. However, the term prase is also used to describe green quartz, and to a certain extent is a color-descriptor, rather than a rigorously defined mineral variety.)
Heliotrope

Heliotrope, or bloodstone
Heliotrope is a green variety of chalcedony, containing red inclusions of iron oxide. These inclusions resemble drops of blood, giving heliotrope its alternative name of bloodstone. A similar variety, in which the spots are yellow instead of red is known as plasma.
Moss agate
Moss agate (also known as tree agate or mocha stone) contains green filament-like inclusions, giving it the superficial appearance of moss or blue cheese. It is not a true form of agate, as it lacks agate’s defining feature of concentric banding.
Mtorolite
Mtorolite is a green variety of chalcedony, which has been colored by chromium. It is principally found in Zimbabwe.
Onyx
Onyx is a variant of agate with black and white banding. Similarly, agate with brown and white banding is known as sardonyx.
History

Chalcedony cameo ofTitushead, 2nd Century AD
As early as the Bronze Age chalcedony was in use in the Mediterranean region; for example, on Minoan Crete at the Palace ofKnossos, chalcedony seals have been recovered dating to circa 1800 BC.[3] People living along the Central Asian trade routes used various forms of chalcedony, including carnelian, to carve intaglios, ring bezels (the upper faceted portion of a gem projecting from the ring setting), and beads that show strong Graeco-Roman influence. Fine examples of first century objects made from chalcedony, possibly Kushan, were found in recent years at Tillya-tepe in north-western Afghanistan. [4] Hot wax would not stick to it so it was often used to make seal impressions. The term chalcedony is derived from the name of the ancient Greek town Chalkedon in Asia Minor, in modern English usually spelledChalcedon, today the Kadıköy district of Istanbul.

Chalcedony knife, AD 1000-1200
Geochemistry
Structure
Chalcedony was once regarded to be a fibrous variety of cryptocrystalline quartz [5]. More recently however, it has been shown to also contain a monoclinic polymorph of quartz, known as moganite[2]. The fraction, by mass, of moganite within a typical chalcedony sample may vary from less than 5% to over 20%[6]. The existence of moganite was once regarded as dubious, but it is now officially recognised by the International Mineralogical Association[7][8].
Solubility
Chalcedony is more soluble than quartz under low-temperature conditions, despite the two minerals being chemically identical. This is thought to be because chalcedony is extremely finely grained (cryptocrystalline), and so has a very high surface area to volume ratio.[citation needed] It has also been suggested that the higher solubility is due to the moganite component [6].
Solubility of quartz and chalcedony in pure water
This table gives equilibrium concentrations of total dissolved silicon as calculated by PHREEQC using the llnl.dat database.
| Temperature |
Quartz Solubility (mg/L) |
Chalcedony Solubility (mg/L) |
| 0.01°C |
0.68 |
1.34 |
| 25.0°C |
2.64 |
4.92 |
| 50.0°C |
6.95 |
12.35 |
| 75.0°C |
14.21 |
24.23 |
| 100.0°C |
24.59 |
40.44 |
|
Amethyst
Amethyst is a violet variety of quartz often used in jewelry. The name comes from the Ancient Greek a- (“not”) and methustos(“intoxicated”), a reference to the belief that the stone protected its owner from drunkenness; the ancient Greeks and Romans wore amethyst and made drinking vessels of it in the belief that it would prevent intoxication.
Chemistry
Amethyst is the violet variety of quartz; its chemical formula is SiO2.
In the 20th century, the color of amethyst was attributed to the presence of manganese. However, since it is capable of being greatly altered and even discharged by heat, the color was believed by some authorities to be from an organic source. Ferric thiocyanatewas suggested, and sulfur was said to have been detected in the mineral.
More recent work has shown that amethysts’ coloration is due to ferric iron impurities.[1]Further study has shown a complex interplay of iron and aluminium is responsible for the color.[2]
On exposure to heat, amethyst generally becomes yellow, and much of the citrine, cairngorm, or yellow quartz of jewelry is said to be merely “burnt amethyst”. Veins of amethystine quartz are apt to lose their color on the exposed outcrop[citation needed].
Synthetic amethyst is made to imitate the best quality amethyst. Its chemical and physical properties are so similar to that of natural amethyst that it can not be differentiated with absolute certainty without advanced gemological testing (which is often cost-prohibitive). There is one test based on “Brazil law twinning” (a form of quartz twinning where right and left hand quartz structures are combined in a single crystal[3]) which can be used to identify synthetic amethyst rather easily. In theory however it is possible to create this material synthetically as well, but this type is not available in large quantities in the market.[4]
Composition
Amethyst is composed of an irregular superposition of alternate lamellae of right-handed and left-handed quartz. It has been shown that this structure may be due to mechanical stresses.
Because it has a hardness of seven on the Mohs scale, amethyst is suitable for use in jewelery.
Hue and tone
Amethyst occurs in primary hues from a light pinkish violet to a deep purple. Amethyst may exhibit one or both secondary hues, red and blue. The ideal grade is called “Deep Siberian” and has a primary purple hue of around 75–80 percent, 15–20 percent blue and (depending on the light source) red secondary hues.[4]

The inside of an AmethystGeode.
History
Amethyst was used as a gemstone by the ancient Egyptians and was largely employed in antiquity for intaglios. The Greeks believed amethyst gems could prevent intoxication, while medieval European soldiers wore amethyst amulets as protection in battle.[citation needed]Beads of amethyst were found in Anglo-Saxon graves in England.[citation needed]
A huge geode, or “amethyst-grotto”, from near Santa Cruz in southern Brazil was exhibited at the Düsseldorf, Germany Exhibition of 1902.
Mythology
The Greek word “amethystos” may be translated as “not drunken”. Amethyst was considered to be a strong antidote against drunkenness, which is why wine goblets were often carved from it. In Greek mythology, Dionysus, the god of intoxication, was pursuing a maiden named Amethystos, who refused his affections.. Amethystos prayed to the gods to remain chaste, which the goddess Artemis granted and transformed her into a white stone. Humbled by Amethystos’s desire to remain chaste, Dionysus poured wine over the stone as an offering, dyeing the crystals purple.
Variations of the story include that Dionysus had been insulted by a mortal and swore to slay the next mortal who crossed his path, creating fierce tigers to carry out his wrath.. The mortal turned out to be a beautiful young woman, Amethystos, who was on her way to pay tribute to Artemis. Her life is spared by Artemis, who transformed the maiden into a statue of pure crystalline quartz to protect her from the brutal claws. Dionysus wept tears of wine in remorse for his action at the sight of the beautiful statue. The god’s tears then stained the quartz purple.[5] Another variation involves the goddess Rhea presenting Dionysus with the amethyst stone to preserve the wine-drinker’s sanity.[6]
Geographic distribution
Amethyst is produced in abundance from the state of Minas Gerais in Brazil where it occurs in large geodes within volcanic rocks. It is also found and mined in South Korea. The largest opencast amethyst vein in the world is in Maissau, Lower Austria. Many of the hollow agates of Brazil and Uruguay contain a crop of amethyst crystals in the interior. Much fine amethyst comes from Russia, especially from near Mursinka in the Ekaterinburg district, where it occurs in drusy cavities in granitic rocks. Many localities in Indiayield amethyst. One of the largest global amethyst producers is Zambia with an annual production of about 1,000 t.

Museum-quality piece of Amethyst
Amethyst occurs at many localities in the United States, but these specimens are rarely fine enough for use in jewelry. Among these may be mentioned Amethyst Mountain, Texas;Yellowstone National Park; Delaware County, Pennsylvania; Haywood County, North Carolina; Deer Hill and Stow, Maine. It is found also in the Lake Superior region. Amethyst is relatively common in Ontario, and in various locations throughout Nova Scotia, but uncommon elsewhere in Canada.
Value
Traditionally included in the cardinal, or most valuable, gemstones (along with diamond,sapphire, ruby, and emerald), amethyst has lost much of its value due to the discovery of extensive deposits in locations such as Brazil. The highest grade amethyst (called “Deep Russian”) is exceptionally rare and therefore its value is dependent on the demand of collectors when one is found. It is however still orders of magnitude lower than the highest grade sapphires or rubies (Padparadscha sapphire or “pigeon’s blood” ruby).[4] |
Quartz
Quartz (from German  Quarz (help·info)[1]) is the second most abundant mineral in the Earth‘s continental crust (after feldspar). It is made up of a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2. Quarz (help·info)[1]) is the second most abundant mineral in the Earth‘s continental crust (after feldspar). It is made up of a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall formula SiO2.
Crystal habit
Quartz belongs to the rhombohedral crystal system. The ideal crystal shape is a six-sided prismterminating with six-sided pyramidsat each end. In nature quartz crystals are often twinned, distorted, or so intergrown with adjacent crystals of quartz or other minerals as to only show part of this shape, or to lack obvious crystal faces altogether and appear massive. Well-formed crystals typically form in a ‘bed’ that has unconstrained growth into a void, but because the crystals must be attached at the other end to a matrix, only one termination pyramid is present. A quartz geode is such a situation where the void is approximately spherical in shape, lined with a bed of crystals pointing inward.
Varieties
Pure quartz, sometimes called clear quartz, is colorless or white and transparent (clear) or translucent. Common colored varieties include citrine, rose quartz, amethyst, smoky quartz, milky quartz, and others. Quartz goes by an array of different names. The most important distinction between types of quartz is that of macrocrystalline (individual crystals visible to the unaided eye) and themicrocrystalline orcryptocrystalline varieties (aggregates of crystals visible only under high magnification). The cryptocrystalline varieties are either translucent or mostly opaque, while the transparent varieties tend to be macrocrystalline. Chalcedony is a cryptocrystalline form of silica consisting of fine intergrowths of both quartz, and its monoclinic polymorph moganite.[2]
Citrine
Citrine is a variety of quartz whose color ranges from a pale yellow to brown. It is nearly impossible to tell cut citrine from yellowtopaz visibly. Citrine has ferric impurities, and is rarely found naturally. Most commercial citrine is in fact artificially heated amethystor smoky quartz. Brazil is the leading producer of citrine, with much of its production coming from the state of Rio Grande do Sul.
Citrine is one of three traditional birthstones for the month of November.
Rose quartz

An elephant carved in rose quartz, 4 inches (10 cm) long
Rose quartz is a type of quartz which exhibits a pale pink to rose red hue. The color is usually considered as due to trace amounts of titanium, iron, or manganese, in the massive material. Some rose quartz contains microscopic rutile needles which produces anasterism in transmitted light. Recent X-ray diffraction studies suggest that the color is due to thin microscopic fibers of possiblydumortierite within the massive quartz.[3]
In crystal form (rarely found) it is called pink quartz and its color is thought to be caused by trace amounts of phosphate oraluminium. The color in crystals is apparently photosensitive and subject to fading. The first crystals were found in a pegmatite found near Rumford, Maine, USA, but most crystals on the market come from Minas Gerais, Brazil..[4]
Rose quartz is not popular as a gem – it is generally too clouded by impurities to be suitable for that purpose. Rose quartz is more often carved into figures such as people or hearts. Hearts are commonly found because rose quartz is pink and an affordable mineral.
Amethyst
Amethyst is a popular form of quartz that ranges from a bright to dark or dull purple/violet color.
Smoky quartz
Smokey quartz is a gray, translucent version of quartz. It ranges in clarity from almost complete transparency to a brownish-grey crystal that is almost opaque.
Milky quartz
Milk quartz or milky quartz may be the most common variety of crystalline quartz and can be found almost anywhere. The white colour may be caused by minute fluid inclusions of gas and/or liquid trapped during the crystal formation. The cloudiness caused by the inclusions effectively bars its use in most optical and quality gemstone applications.[5]
Major varieties
Although many of the varietal names historically arose from the color of the mineral, current scientific naming schemes refer primarily to the microstructure of the mineral. Color is a secondary identifier for the cryptocrystalline minerals, although it is a primary identifier for the macrocrystalline varieties. This does not always hold true.
Major Varieties of Quartz
| Chalcedony |
Cryptocrystalline quartz and moganite mixture. The term is generally only used for white or lightly colored material. Otherwise more specific names are used. |
| Agate |
Multi-colored, banded chalcedony, semi-translucent to translucent |
| Onyx |
Agate where the bands are straight, parallel and consistent in size. |
| Jasper |
Opaque cryptocrystalline quartz, typically red to brown |
| Aventurine |
Translucent chalcedony with small inclusions (usually mica) that shimmer. |
| Tiger’s eye |
Fibrous gold to red-brown coloured quartz, exhibiting chatoyancy. |
| Rock crystal |
Clear, colorless |
| Amethyst |
Purple, transparent |
| Citrine |
Yellow to reddish orange to brown, greenish yellow |
| Prasiolite |
Mint green, transparent |
| Rose quartz |
Pink, translucent, may display diasterism |
| Rutilated quartz |
Contains acicular (needles) inclusions of rutile |
| Milk quartz |
White, translucent to opaque, may display diasterism |
| Smoky quartz |
Brown to grey, opaque |
| Carnelian |
Reddish orange chalcedony, translucent |
Synthetic and artificial treatments

A synthetic quartz crystal grown by the hydrothermal method, about 19 cm long and weighing about 127 grams.
Not all varieties of quartz are naturally occurring. Prasiolite, an olive colored material, is produced by heat treatment; natural prasiolite has also been observed in Lower Silesia in Poland. Although citrine occurs naturally, the majority is the result of heat-treated amethyst. Carnelian is widely heat-treated to deepen its color.
Due to natural quartz being so often twinned, much of the quartz used in industry is synthesized. Large, flawless and untwinned crystals are produced in an autoclave via the hydrothermal process; emeralds are also synthesized in this fashion. While these are still commonly referred to as quartz, the correct term for this material is silicon dioxide.
Occurrence
Quartz is an essential constituent of granite and other felsic igneous rocks. It is very common insedimentary rocks such assandstone and shale and is also present in variable amounts as an accessory mineral in most carbonate rocks. It is also a common constituent of schist, gneiss, quartzite and othermetamorphic rocks. Because of its resistance to weathering it is very common in stream sediments and in residual soils.
Quartz occurs in hydrothermal veins as gangue along with ore minerals. Large crystals of quartz are found in pegmatites. Well-formed crystals may reach several meters in length and weigh hundreds of kilograms.
Related silica minerals
Tridymite and cristobalite are high-temperature polymorphs of SiO2 that occur in high-silica volcanic rocks.Coesite is a denser polymorph of quartz found in some meteorite impact sites and in metamorphic rocks formed at pressures greater than those typical of the Earth’s crust. Stishovite is a yet denser and higher-pressure polymorph of quartz found in some meteorite impact sites.Lechatelierite is an amorphous silicaglass SiO2 which is formed by lightning strikes in quartz sand.
History
The name “quartz” comes from the German “Quarz”, which is of Slavic origin (Czech miners called itkřemen). Other sources insist the name is from the Saxon word “Querkluftertz”, meaning cross-vein ore.[6]
Quartz is the most common material identified as the mystical substance maban in Australian Aboriginal mythology. It is found regularly in passage tomb cemeteries in Europe in a burial context, eg. Newgrange orCarrowmore in the Republic of Ireland. The Irishword for quartz is grian cloch, which means ‘stone of the sun’.
Roman naturalist Pliny the Elder believed quartz to be water ice, permanently frozen after great lengths of time. (The word “crystal” comes from the Greek word for purity.) He supported this idea by saying that quartz is found near glaciers in the Alps, but not on volcanic mountains, and that large quartz crystals were fashioned into spheres to cool the hands. He also knew of the ability of quartz to split light into a spectrum. This idea persisted until at least the 1600s.
In the 17th century, Nicolas Steno‘s study of quartz paved the way for modern crystallography. He discovered that no matter how distorted a quartz crystal, the long prism faces always made a perfect 60 degree angle.
Charles Sawyer invented the commercial quartz crystal manufacturing process in Cleveland, Ohio, United States. This initiated the transition from mined and cut quartz for electrical appliances to manufactured quartz.
Quartz’s piezoelectric properties were discovered by Jacques and Pierre Curie in 1880. The quartz oscillatoror resonator was first developed by Walter Guyton Cady in 1921.[7] George Washington Pierce designed and patented quartz crystal oscillators in 1923.[8]Warren Marrison created the first quartz oscillator clock based on the work of Cady and Pierce in 1927.[9]
Piezoelectricity
Quartz crystals have piezoelectric properties; they develop an electric potential upon the application ofmechanical stress. An early use of this property of quartz crystals was in phonograph pickups. One of the most common piezoelectric uses of quartz today is as a crystal oscillator. The quartz clock is a familiar device using the mineral. The resonant frequency of a quartz crystal oscillator is changed by mechanically loading it, and this principle is used for very accurate measurements of very small mass changes in thequartz crystal microbalance and in thin-film thickness monitors. |
Spodumene
- “Kunzite” redirects here. For the Sailor Moon character, see Shitennou.
| Spodumene |

An almost colorless kunzite crystal (upper left), a cut pale pink kunzite (upper right) and a greenish hiddenite crystal (below) (unknown scale) |
| General |
| Category |
Mineral |
| Chemical formula |
lithium aluminium silicate, LiAl(SiO3)2 |
| Identification |
| Color |
Highly variable: white, colorless, gray, pink, lilac, violet, yellow and green |
| Crystal habit |
prismatic, generally flattened and elongated |
| Crystal system |
monoclinic; 2/m |
| Cleavage |
Perfect prismatic, two directions at nearly 90° |
| Fracture |
Sub-conchoidal |
| Mohs Scalehardness |
6.5 – 7 |
| Luster |
Vitreous |
| Streak |
white |
| Specific gravity |
3.17-3.19 |
| Refractive index |
1.66-1.68 |
| Pleochroism |
Strong in kunzite: pink, colorless; hiddenite: yellow-green, blue-green |
| Fusibility |
3.5 |
| Solubility |
insoluble |
| Other characteristics |
Tenebrescence, chatoyancy, kunzite often fluorescentunderUV |
Spodumene is a pyroxene mineral consisting of lithium aluminium inosilicate - LiAl(SiO3)2 – and is a source of lithium. It occurs as colorless to yellowish, purplish or lilac kunzite (see below), yellowish-green or emerald-green hiddenite, prismatic crystals, often of great size. Single crystals of 14.3 m in size are reported from the Black Hills of South Dakota, United States.[1]
Crystals form in the monoclinic system and are typically heavily striated parallel to the principal axis. Crystal faces are often etched and pitted with triangular markings.
Spodumene is derived from the Greek spodumenos (σποδυμενος), meaning “burnt to ashes,” owing to the opaque, ash-grey appearance of material refined for use in industry.
Spodumene occurs in lithium rich granites and pegmatites. Transparent material has long been used as a gemstone with varieties kunzite and hiddenite noted for their strongpleochroism. Source localities include Afghanistan, Australia, Brazil, Madagascar,Pakistanand USA (North Carolina, California).
Economic importance
Spodumene is an important source of lithium for use in ceramics, mobile phone andautomotive batteries, medicine and as a fluxing agent. Lithium is extracted from spodumene by fusing in acid.
World production of lithium via spodumene is around 80,000 metric tonnes per annum, primarily from the Greenbushes pegmatite ofWestern Australia, and some Chinese andChilean sources. The Talison mine in Greenbushes, Western Australia has an estimated reserve of 13 million tonnes.[2]
Spodumene is becoming less important a source of lithium due to the emergence of alkalinebrine lake sources in Chile, China andArgentina, which produce lithium chloride directly. Lithium chloride is converted to lithium carbonate and lithium hydroxide by reaction withsodium carbonate and calcium hydroxide respectively.
Kunzite

Kunzite
See the pleochroism and the typical etched marks (unknown scale)
Kunzite is a pink to lilac colored gemstone, a variety of spodumene with the color coming from minor to trace amounts ofmanganese. Some (but not all) kunzite used for gemstones has been heated to enhance its color. It is also frequently irradiated to enhance the color. Many kunzites fade when exposed to sunlight. It was discovered in 1902, and was named after George Frederick Kunz, Tiffany & Co‘s chief jeweler at the time, and a noted mineralogist. It has been found in Brazil, USA, Canada, CIS, Mexico,Sweden, Western Australia, Afghanistan and Pakistan.
Tourmaline
| Tourmaline |

Schorl Tourmaline |
| General |
| Category |
Silicate mineral group |
| Chemical formula |
(Ca,K,Na,[])(Al,Fe,Li,Mg,Mn)3(Al,Cr, Fe,V)6
(BO3)3(Si,Al,B)6O18(OH,F)4
[1][2] |
| Identification |
| Color |
Most commonly black, but can range from brown, violet, green, pink, or in a dual-colored pink and green. |
| Crystal habit |
Parallel and elongated. Acicular prisms, sometimes radiating. Massive. Scattered grains (in granite). |
| Crystal system |
Trigonal |
| Cleavage |
Indistinct |
| Fracture |
Uneven, small conchoidal, brittle |
| Mohs Scalehardness |
7–7.5 |
| Luster |
Vitreous, sometimes resinous |
| Streak |
White |
| Specific gravity |
3.06 (+.20 -.06)[1] |
| Density |
2.82–3.32 |
| Polish luster |
Vitreous[1] |
| Optical properties |
Double refractive, uniaxial negative[1] |
| Refractive index |
nω=1.635–1.675, nε=1.610–1.650 |
| Birefringence |
-0.018 to -0.040; typically about .020 but in dark stones it may reach .040[1] |
| Pleochroism |
typically moderate to strong[1]
Red Tourmaline: Definite; dark red,light red
Green Tourmaline: Strong; dark green, yellow-green
Brown Tourmaline: Definite; dark brown, light brown
Blue Tourmaline: Strong; dark blue, light blue |
| Dispersion |
.017[1] |
| Ultravioletfluorescence |
pink stones—inert to very weak red to violet in long and short wave[1] |
| Absorption spectra |
a strong narrow band at 498nm, and almost complete absorption of red down to 640nm in blue and green stones; red and pink stones show lines at 458 and 451nm as well as a broad band in the green spectrum[1] |
Tourmaline is a crystal silicate mineral compounded with elements such as aluminium, iron,magnesium, sodium, lithium, orpotassium. Tourmaline is classed as a semi-precious stone and the gem comes in a wide variety of colors. The name comes from the Sinhalese word “turamali” or “toramalli”, which applied to different gemstones found in Ceylon (now Sri Lanka).
History
Brightly colored Sri Lankan gem tourmalines were brought to Europe in great quantities by theDutch East India Company to satisfy a demand for curiosities and gems. At the time it was not realised that schorl and tourmaline were the same mineral.
Tourmaline species and varieties
- Dravite species: from the Drave district of Carinthia
- Dark yellow to brownish black—dravite
- Schorl Species:
- Bluish or brownish black to Black—schorl
- Elbaite Species: named after the island of Elba, Italy
- Rose or pink—rubellite variety(from ruby)
- Dark black—schorl(from indigo)
- Light blue to bluish green—Brazilian indicolite variety
- Green—verdelite or Brazilian emerald variety
- Colorless—achroite variety (from the Greek for “colorless”)
Schorl
The most common species of tourmaline is schorl. It may account for 95% or more of all tourmaline in nature. The early history of the mineral schorl shows that the name “schorl” was in use prior to 1400 AD because a village known today as Zschorlau (inSaxony, Germany) was then named “Schorl” (or minor variants of this name). This village had a nearby tin mine where, in addition tocassiterite, black tourmaline was found. The first description of schorl with the name “schürl” and its occurrence (various tin mines in the Saxony Ore Mountains) was written by Johannes Mathesius (1504–1565) in 1562 under the title “Sarepta oder Bergpostill” (Ertl, 2006). Up to about 1600, additional names used in the German languagewere “Schurel”, “Schörle”, and “Schurl”. From the 18th century on, the name “Schörl” was mainly used in the German-speaking area. In English, the names “shorl” and “shirl” were used in the 18th century for schorl. In the 19th century the names “common schorl”, “schörl”, “schorl” and “iron tourmaline” were used in the Anglo-Saxon area (Ertl, 2006). The word tourmaline has two etymologies, both from the Sinhalese word turamali, meaning “stone attracting ash” (a reference to its pyroelectric properties) or according to other sources “mixed gemstones”.
Dravite
The name dravite was used for the first time by Gustav Tschermak (1836 – 1927; Professor of Mineralogy and Petrography at the University of Vienna) in his book “Lehrbuch der Mineralogie” (published in 1884) for Mg-rich (and Na-rich) tourmaline from the village Unterdrauburg, Drava river area, Carinthia, Austro-Hungarian Empire. Today this tourmaline locality (type locality for dravite) at the village Dravograd (near Dobrova pri Dravogradu), is a part of the Republic of Slovenia (Ertl, 2007). Tschermak gave this tourmaline the name dravite, for the Drava river area, which is the district along the Drava River (in German: Drau, in Latin: Drave) in Austria and Slovenia. The chemical composition which was given by Tschermak in 1884 for this dravite approximately corresponds to the formula NaMg3(Al,Mg)6B3Si6O27(OH), which is in good agreement (except for the OH content) with the endmember formula of dravite as known today (Ertl, 2007).
Elbaite
A lithium-tourmaline (elbaite) was one of three pegmatitic minerals from Utö, Sweden, in which the new alkali element lithium (Li) was determined in 1818 by Arfwedson for the first time (Ertl, 2008). Elba Island, Italy,was one of the first localities where colored and colorless Li-tourmalines were extensively chemically analysed. In 1850 Rammelsberg described fluorine in tourmaline for the first time. In 1870 he proved that all varieties of tourmaline contain chemically bound water. In 1889 Scharitzer proposed the substitution of (OH) by F in red Li-tourmaline from Sušice, Czech Republic. In 1914 Vernadsky proposed the name “Elbait” for Li-,Na-, and Al-rich tourmaline from Elba Island, Italy, with the simplified formula (Li,Na)HAl6B2Si4O21 (Ertl, 2008). Most likely the type material for elbaite was found at Fonte del Prete, San Piero in Campo, Campo nell’Elba, Elba Island, Livorno Province, Tuscany, Italy (Ertl, 2008). In 1933 Winchell published an updated formula for elbaite, H8Na2Li3Al3B6Al12Si12O62, which is commonly used to date written as Na(Li1.5Al1.5)Al6(BO3)3[Si6O18](OH)3(OH) (Ertl, 2008). The first crystal structure determination of a Li-rich tourmaline was published in 1972 by Donnay and Barton, performed on a pink elbaite from San Diego County, California, U.S.A.
Chemical composition tourmaline group
The tourmaline mineral group is chemically one of the most complicated groups of silicate minerals. Its composition varies widely because of isomorphous replacement (solid solution), and its general formula can be written as
XY3Z6(T6O18)(BO3)3V3W,
where:[3]
X = Ca, Na, K, vacancy
Y = Li, Mg, Fe2+, Mn2+, Zn, Al, Cr3+, V3+, Fe3+, Ti4+, vacancy
Z = Mg, Al, Fe3+, Cr3+, V3+
T = Si, Al, B
B = B, vacancy
V = OH, O
W = OH, F, O

Tri-color elbaite crystals on quartz, Himalaya Mine, San Diego Co., California, USA
Physical properties
Crystal structure
Tourmaline belongs to the trigonal crystal system and occurs as long, slender to thick prismatic and columnar crystals that are usually triangular in cross-section. The style of termination at the ends of crystals is asymmetrical, called hemimorphism. Small slender prismatic crystals are common in a fine-grained granite called aplite, often forming radial daisy-like patterns. Tourmaline is distinguished by its three-sided prisms; no other common mineral has three sides. Prisms faces often have heavy vertical striations that produce a rounded triangular effect. Tourmaline is rarely perfectly euhedral. An exception was the fine dravite tourmalines ofYinnietharra, in western Australia. The deposit was discovered in the 1970s, but is now exhausted. All hemimorphic crystals arepiezoelectric, and are oftenpyroelectric as well.
Color
Tourmaline has a variety of colors. Usually, iron-rich tourmalines are black to bluish-black to deep brown, while magnesium-rich varieties are brown to yellow, and lithium-rich tourmalines are almost any color: blue, green, red, yellow, pink etc. Rarely, it is colorless. Bi-colored and multicolored crystals are common, reflecting variations of fluid chemistry during crystallisation. Crystals may be green at one end and pink at the other, or green on the outside and pink inside: this type is called watermelon tourmaline. Some forms of tourmaline are dichroic, in that they change color when viewed from different directions.

Large pink elbaite crystal on quartz, Cryo-Genie Mine, San Diego Co., California, USA.
Treatments
Some tourmaline gems, especially pink to red colored stones, are altered by irradiation to improve their color. Irradiation is almost impossible to detect in tourmalines, and does not impact the value. Heavily-included tourmalines, such as rubellite and Brazilian paraiba, are sometimes clarity enhanced. A clarity-enhanced tourmaline (especially paraiba) is worth much less than a non-treated gem.[4]
Geology
Tourmaline is found in two main geological occurrences. Igneous rocks, in particular graniteand granite pegmatite and inmetamorphic rocks such as schist and marble. Schorl and lithium-rich tourmalines are usually found in granite and granitepegmatite. Magnesium-rich tourmalines, dravites, are generally restricted to schists and marble. Tourmaline is a durable mineral and can be found in minor amounts as grains in sandstone and conglomerate.

Bi-colored tourmaline crystal, 0.8 inches long (2 cm).
Tourmaline localities
Gem and specimen tourmaline is mined chiefly in Brazil and Africa. Some placer material suitable for gem use comes from Sri Lanka. In addition to Brazil, tourmaline is mined inTanzania, Nigeria, Kenya, Madagascar, Mozambique, Namibia, Afghanistan,Pakistan, andSri Lanka, and Malawi.[5]
United States
Some fine gems and specimen material has been produced in the United States, with the first discoveries in 1822, in the state ofMaine. California became a large producer of tourmaline in the early 1900s. The Maine deposits tend to produce crystals in raspberry pink-red as well as minty greens. The California deposits are known for bright pinks, as well as bicolors. During the early 1900s, Maine and California were the world’s largest producers of gem tourmalines. The Empress Dowager Tz’u Hsi, the last Empress ofChina, loved pink tourmaline and bought large quantities for gemstones and carvings from the then new Himalaya Mine, located inSan Diego County, California. [6] It is not clear when the first tourmaline was found in California.Native Americans have used pink and green tourmaline as funeral gifts for centuries. The first documented case was in 1890 when Charles Russel Orcutt found pink tourmaline at what later became the Stewart Mine at Pala, San Diego [7].
Brazil

Watermelon Tourmaline mineral on quartz matrix (crystal approximately 2 cm wide at face)
Almost every color of tourmaline can be found in Brazil, especially in the Brazilian states ofMinas Gerais and Bahia. In 1989, miners discovered a unique and brightly colored variety of tourmaline in the state of Paraíba. The new type of tourmaline, which soon became known as paraiba tourmaline, came in unusually vivid blues and greens. These colors were often described as “neon” since they appeared to glow. Brazilian paraiba tourmaline is usually heavily included. Much of the paraiba tourmaline from Brazil actually comes from the neighboring state of Rio Grande do Norte. Material from Rio Grande do Norte is often somewhat less intense in color, but many fine gems are found there. It was determined that the element copper was important in the coloration of the stone.
Africa
In the late 1990s, copper-containing tourmaline was found in Nigeria. The material was generally paler and less saturated than the Brazilian materials, although the material generally was much less included. A more recent African discovery from Mozambique has also produced beautiful tourmaline colored by copper, similar to the Brazilian paraiba. While its colors are somewhat less bright than top Brazilian material, Mozambique paraiba is often less included and has been found in larger sizes. The Mozambique paraiba material usually is more intensely colored than the Nigerian. There is a significant overlap in color and clarity with Mozambique paraiba and Brazilian paraiba, especially with the material from Rio Grande do Norte. While less expensive than top quality Brazilian paraiba, some Mozambique material sells for well over $5,000 per carat, which still is extremely high compared to other tourmalines.

Tourmaline mineral (approximately 10 cm tall)
Another highly valuable variety is chrome tourmaline, a rare type of dravite tourmaline fromTanzania. Chrome tourmaline is a rich green color due to the presence of chromium atoms in the crystal; chromium also produces the green color of emeralds. Of the standard elbaite colors, blue indicolite gems are typically the most valuable, followed by green verdelite and pink to red rubellite.[citation needed] There are also yellow tourmalines, sometimes known as canary tourmaline. Zambia is rich in both red and yellow tourmaline, which are relatively inexpensive in that country. Ironically the rarest variety, colorless achroite, is not appreciated and is the least expensive of the transparent tourmalines.
Afghanistan
In the Nuristan region of Kunar province extra fine Indicolite (blue tourmaline) and Verderite (green tourmaline) are found. Gem quality Tourmaline are faceted (cut) from 0.50-10 gram sizes and have unusually high clarity and intense shades of color
Zircon

Crystal structure of zircon
Zircon (including hyacinth or yellow zircon) is a mineral belonging to the group ofnesosilicates. Its chemical name iszirconium silicate and its corresponding chemical formula is ZrSiO4. Hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, and green. Colorless specimens that show gem quality are a popular substitute fordiamond; these specimens are also known as “Matura diamond”. It is not to be confused with cubic zirconia, a synthetic substance with a completely different chemical composition.
The name either derives from the Arabic word zarqun, meaning vermilion, or from the Persianzargun, meaning golden-colored. These words are corrupted into “jargoon“, a term applied to light-colored zircons. Yellow zircon is called“hyacinth”, from the flower hyacinthus, whose name is of Ancient Greek origin; in the Middle Ages all yellow stones of East Indian origin were called hyacinth, but today this term is restricted to the yellow zircons.
Zircon is regarded as the traditional birthstone for December.
Properties

Optical microscope photograph; the length of the crystal is about 250µm.
Zircon is a remarkable mineral, if only for its almost ubiquitous presence in the crust of Earth. It occurs in igneous rocks (as primary crystallization products), in metamorphic rocks and insedimentary rocks (as detrital grains). Large zircon crystals are seldom abundant. Their average size, e.g. in granite rocks, is about 100–300 µm, but they can also grow to sizes of several centimeters (a few inches), especially in pegmatites.
Owing to their uranium and thorium content, some zircons may undergo metamictization. The processes, related to internal radiation damage, partially disrupt the crystal structure and partly explain the highly-variable properties of zircon. As zircon becomes more and more modified by internal radiation damage, the density decreases, the crystal structure is compromised, and the color changes.
Zircon is a common accessory mineral that occurs worldwide. Noted occurrences include:Australia; Russia (Ural Mountains); Trentino, Monte Somma, and Vesuvius, Italy; Arendal,Norway; Sri Lanka; India; Indonesia , Java, Kalimantan,Sulawesi; Thailand; Ratanakiri,Cambodia; the Kimberley mines, Republic of South Africa; Madagascar; Renfrew County,Ontario, and Grenville, Quebec, Canada; and Litchfield, Maine; Chesterfield, Massachusetts; Essex, Orange, and St. Lawrence counties, New York; Henderson County, North Carolina; thePikes Peak district of Colorado; and Llano County, Texas in the United States. Australia leads the world in zircon mining, producing 37% of the world total and accounting for 40% of world EDR (economic demonstrated resources) for the mineral. Thorite (ThSiO4) is an isostructural related mineral.
Zircon occurs in many different colors, including red, pink, brown, yellow, hazel, black, or colorless. The color of zircons can be changed by heat treatment. Depending on the amount of heat applied, colorless, blue, and golden-yellow zircons can be made. In geological settings, the development of pink, red, and purple zircon occurs after 100′s of millions of years provided the crystal has sufficient trace elements to produce color centers. Color in this red or pink series is annealed in geological conditions above about 350°C.
Uses

Sand-sized grains of zircon
- Zircons are commercially mined for the metal zirconium, and are used for abrasive and insulating purposes.
- It is the source of zirconium oxide(ZrO2), one of the most refractory materials known.
- Crucibles of ZrO2 are used to fuse platinum at temperatures in excess of 1755 oC.
- Zirconium metal is used in nuclear reactors due to its neutron absorption properties.
- Large specimens are appreciated as gemstones, owing to their high refractive index. (Zircon has a refractive index of approximately 1.95; diamond‘s is approximately 2.4.)
- Zircon is one of the key minerals used by geologists for geochronology .
Occurrence

World production trend of zirconium mineral concentrates
Zircon is a common accessory to trace mineral constituent of most granite and felsic igneous rocks. Due to its hardness, durability and chemical inertness, zircon persists in sedimentary deposits and is a common constituent of most sands. Zircon is rare within mafic rocks and very rare within ultramafic rocks aside from a group of ultrapotassic intrusive rocks such askimberlites, carbonatites, and lamprophyre, where zircon can occasionally be found as a trace mineral owing to the unusual magma genesis of these rocks.
Zircon forms economic concentrations within heavy mineral sands ore deposits, within certainpegmatites, and within some rare alkaline volcanic rocks, for example the Toongi Trachyte, Dubbo, New South Wales Australia[1] in association with the zirconium-hafnium mineralseudialyte and armstrongite.
Zircons and radiometric dating
Zircon has played an important role during the evolution of radiometric dating. Zircons contain trace amounts of uraniumand thorium (from 10 ppm up to 1 wt%) and can be dated using several modern analytical techniques. Because zircons can survive geologic processes likeerosion, transport, even high-grade metamorphism, they contain a rich and varied record of geological processes. Currently, zircons are typically dated by U/Pb, fission-track dating, and U+Th/He dating techniques.
The oldest minerals dated so far by the U/Pb technique are zircons from Jack Hills in theNarryer Gneiss Terrane, Yilgarn Craton, Western Australia, with an age of 4.404 billion years,[2] interpreted to be the age of crystallization. These zircons might be the oldest minerals on earth. In addition, the oxygen isotopic composition has been interpreted to indicate that more than 4.4 billion years ago there was already water on the surface of the Earth. This interpretation has been published in scientific journals[3][4] but is the subject of debate.[citation needed]
Zircons and Oxygen Isotope Dating
Oxygen isotope dating is also used to help to identify the age of zircons in many metamorphic, igneous, and clastic sedimentary rocks. Unlike some minerals, zircons are able to preserve oxygen isotope ratios through many environmental processes, even through metamorphism and hydrothermal alteration.. The oxygen ratio of oxygen-18 to oxygen-16 is also a way of helping to better understand the type of atmospheric conditions when the minerals were being formed. Zircons that date back to the Archean are found in the Canadian Shield, and an area where zircons are extracted heavily for dating.[5]
Grossular
Grossular or grossularite is a calcium-aluminium mineral species of the garnet group with the formula Ca3Al2(SiO4)3,[1] though the calcium may in part be replaced by ferrous iron and the aluminium by ferric iron. The name grossular is derived from the botanicalname for thegooseberry, grossularia, in reference to the green garnet of this composition that is found inSiberia. Other shades include cinnamon brown (cinnamon stone variety), red, and yellow.
The more common variety of grossular is called hessonite from the Greek meaning inferior, because of its inferior hardness to zircon, which the yellow crystals resemble. Grossular is found in contact metamorphosed limestones with vesuvianite, diopside, wollastoniteandwernerite.
A highly sought after variety of gem garnet is the fine green Grossular garnet from Kenya andTanzania called tsavorite. This garnet was discovered in the 1960s in the Tsavo area of Kenya, from which the gem takes its name.

Orange Grossular garnet crystals from a contact metamorphic deposit, Nevada, USA
Viluite is a variety name of grossular, that is not a recognized mineral species.[3] It is usually olive green though sometimes brownish or reddish, brought about by impurities in the crystal. Viluite is found associated with and is similar in appearance to vesuvianite, and there is confusion in terminology as viluite has long been used as a synonym for wiluite, a sorosilicateof the vesuvianite group. This confusion in nomenclature dates back to James Dwight Dana.[4]It comes from the Vilyuy river area in Siberia.
Grossular is known by many other names, and also some misnomers;[5] colophonite – coarse granules of garnet[6], ernite,gooseberry-garnet – light green colored and translucent,[7]kalkthongranat, kanelstein, olyntholite/olytholite, pechgranat, romanzovite, and tellemarkite. Misnomers include;[2] South African jade, garnet jade, Transvaal jade, and African jade. |
Aquamarine – A quality stone
Aquamarine Be3Al3 (SiO3) 6 is a gemstone of transparent variety of beryl. It has a soft blue or blue green color. It is suggestive of the tint of sea water, as blue as the Caribbean. It is related closely to gem emerald. Beryl is a mineral made of beryllium aluminum silicate, a marketable source of beryllium. Aquamarine is the most familiar variety of gem beryl. it appears in pegmatite, an igneous rock. There, it becomes really large and clear crystals than the emerald. Aquamarine has a hexagonal crystal structure and chemical formula of Be3Al2Si6O18, a beryllium Aluminium silicate mineral.

Aquamarine gemstone has long been of great interest because it has many varieties that are valued in a gemstone. Though Aquamarine belongs to the same family as the emerald, but emeralds are much brittle in comparison. Along with bloodstone, Aquamarine is a birthstone of Pisces birth sign (Fish) (Feb. 19 – March 20).
Aquamarine Etymology
Aquamarine has had its name in an interesting way. its name comes from Latin language, aqua marina, meaning, “water of the sea”. It is called as heliodor; rose pink beryl, Morganite, white beryl and goshenite.
Historical Qualities and lore
Since the ancient time, Aquamarine is credited with a stone that offers courage, treatment for laziness and reviving the intellect. It was called the treasure of mermaids having a great power to keep sailors at sea safe. It is said to have a peculiarly strong charm when it is kept immersed in water the power that sailors need the most.
The ancient people used to believe that Aquamarine is said to have a great pacifying influence on land, on the married couples particularly. Such was its power that helped husbands and wives to work out their differences. It ensures that the couple has a long and happy marriage. Thus, it is a best anniversary gift for the love birds!
Aquamarine allegedly protects the wearer against the wiles of devil. A dream of aquamarine had the significance that you will meet new friends. People believed Aquamarine can reawaken love not only in long married couples but also in the new friends.
Others reportedly say that Aquamarine is the right stone for meditation. In middle ages, Aquamarine was believed to impart the wearer with great insight, foresight and freedom from insomnia. From any years, it was credited with great reputation of offering its wearers with happiness and eternal youth.
Aquamarine Color
The colors of Aquamarine are great and charming. You can find them in a wide range of blue shades. They range from the palest pastel to greenish blue to deep blue. The deep blue gems are, in fact, rare. Aquamarine is pastel gemstone whose color can be greatly intense in large gemstones. A small Aquamarine stone can often be less vivid. The choice of color depends more on your taste and preference. Mostly natural Aquamarine is cool greenish blue.
The iron impurities are located in the beryl crystal structure. The color of Aquamarine is mainly because of hint amounts of iron impurities in beryl structure. The different colors of Aquamarine depend on the concentration of iron in the stone.
The jewelry industry has devised many advanced methods to improvise the color of Aquamarine gemstone. The modern methods get Aquamarine heat treated to drive the color out of the stone. It then leaves a much pleasing dark blue color. The process can be reversed through irradiation. The yellow and green colors can be thus restored. Generally, it calls for a stone of a definite size to have a dark shade.
The treatment methods of Aquamarine stones are mostly a permanent treatment. Though a dark and pure blue aquamarine stone has become desirable these days, but if you prefer a little natural green gems, you can find that they are not heat treated and mostly less expensive. The Aquamarine having dark blue color is often the most valuable, but the color generally ranges from light turquoise blue to bluish green. The Aquamarine stone with green color is less valuable and thus cheaper than other stones.
Remedial qualities
Aquamarine is best against all sorts of nerve pain, gland diseases, toothache and troubles in the jaw, neck and throat. It gives strength to liver and kidneys. Aquamarine reduces problems of eyes, ears and stomach. It also relieves cough. Aquamarine defends the wearer from perils of the sea, as well as seasickness. It can help to ease depression and pain.
Aquamarine Sizes and price
Aquamarine is found in many sizes and qualities. Gems are cut that weigh about many hundred carats. They are too large to be worn by anyone. Thus, the price of aquamarine gemstone doesn’t differ in sizes more than a carat. Aquamarine is generally worth the same price for each carat as a one carat gem of good quality. Its price depends on its clarity and the profoundness of color.
Shopping Tips-How to find Aquamarine with good qualities
Aquamarine is a remarkable and affordable gemstone of great quality. aquamarine is a transparent and hard gemstone that makes it a great choice for beautiful jewelry such as rings, earrings or pendants. With its delicate color and perfect clarity, Aquamarine is best exhibited in a better prominent mold.
For many years, aquamarine is the favorite gemstone of many consumers. When you go out in the market to choose high quality aquamarine for yourself, it may become a bit difficult for you. you may get very confused as to how to decide about the qualities of Aquamarine. Here are some worthy tips-
1. Buy Aquamarine only with a reputable and reliable jeweler. The jeweler must be well familiar with aquamarine in particular and also gemstones in general. They are the only people who may sell Aquamarine of real good quality.
2. To make out the best quality of Aquamarine, you must ask if the stone is treated in any way. the common treatments can be heat treatment, irradiation, coating and dyeing. Though not all the treatments may devaluate the stone, you must always ask the seller about the qualities of Aquamarine what you are buying.
3. Check the color, clarity and cut of the stone. Look at the stone from all different angles. Aquamarine displays a light pastel color. You can check that the light reflects evenly from the surface of gem. There must not be any scratches on the gemstone. Only, after a close look at all the features of the stone you must conclude about the quality of the stone. |
Saturday, July 18, 2009
Beryl
“Heliodor” redirects here. For the masculine name, see Heliodorus. The mineral beryl is a beryllium aluminium cyclosilicate with the chemical formulaBe3Al2(SiO3)6. The hexagonal crystals of beryl may be very small or range to several meters in size.. Terminated crystals are relatively rare. Pure beryl is colorless, but it is frequently tinted by impurities; possible colors are green, blue, yellow, red, and white. The name comes from the Greek berylloswhich referred to a precious blue-green color-of-sea-water stone.[1]The term was later adopted for the mineral beryl more exclusively.[2]
Deposits
Beryl of various colors is found most commonly in granitic pegmatites, but also occurs in micaschists in the Ural Mountains, andlimestone in Colombia. Beryl is often associated with tinand tungsten ore bodies. Beryl is found in Europe in Norway, Austria,Germany, and Ireland, as well as Brazil, Colombia, Madagascar, Sweden (especially morganite), Russia, South Africa, the United States, and Zambia. U.S. beryl locations are in California, Colorado, Idaho,Maine, Connecticut, New Hampshire, North Carolina,South Dakota, and Utah.
The most famous source of emeralds in the world is at Muzo and Chivor, Boyacá, Colombia, where they make a unique appearance in limestone. Emerald are also found in the Transvaal, South Africa; Minas Gerais, Brazil; Zambia, and near Mursinka in the Ural Mountains in Russia. In the United States, emeralds are found in North Carolina.
New England‘s pegmatites have produced some of the largest beryls found, including one massive crystal from the Bumpus Quarry in Albany, Maine with dimensions 5.5 m by 1.2 m (18 ft by 4 ft) with a mass of around 18 metric tons; it is New Hampshire’s state mineral. As of 1999, the largest known crystal of any mineral in the world is a crystal of beryl from Madagascar, 18 meters long and 3.5 meters in diameter.[3]
Varieties
Varieties of beryl have been considered gemstones since prehistoric times:
Morganite
Morganite, also known as “pink beryl,” “rose beryl,” “pink emerald,” and “cesian beryl,” is a rare light pink to rose-colored gem-quality variety of beryl. Orange/yellow varieties of morganite can also be found, and color banding is common. It can be routinely heat treated to remove patches of yellow and is occasionally treated by irradiation to improve its color. The pink color of morganite is attributed to Mn2+ ions.[4]
Discovery and naming
Morganite was first discovered together with other gemstone minerals, such as tourmaline andkunzite, at Pala, California, early in the twentieth century. This started a bonanza for these quite popular gemstones which drew the attention of gemologist George Frederick Kunz, who knew that pink beryl was quite a rarity. [5] In 1911, Kunz suggested naming the pink variety of beryl “morganite” after financier J. P. Morgan.[6]
The Rose of Maine
On October 7, 1989, one of the largest gem morganite specimens ever uncovered, eventually called “The Rose of Maine,” was found at the Bennett Quarry in Buckfield, Maine.[7] The crystal, originally somewhat orange in hue, was 23 cm long and about 30 cm across, and weighed (along with its matrix) just over 50 lbs (23 kg).[8]
Bixbite
Red beryl (also known as “bixbite”, “red emerald”, or “scarlet emerald”) is a red variety of beryl. It was first described in 1904 for an occurrence, its type locality, at Maynard’s Claim (Pismire Knolls), Thomas Range, Juab County, Utah, USA.[9][10] The old synonym bixbite is deprecated from the CIBJO, because of the risk of confusion with the mineral bixbyite (also named after the mineralogist Maynard Bixby). The dark red color of bixbite is attributed to Mn3+ ions.[4]
Red beryl is rare and has only been reported from a handful of locations including: Wah Wah Mountains, Beaver County, Utah; Paramount Canyon,Sierra County, New Mexico; Round Mountain, Sierra County, New Mexico; andJuab County, Utah. The greatest concentration of gem-grade red beryl comes from the Violet Claim in the Wah Wah Mountains of mid-western Utah, discovered in 1958 by Lamar Hodges, of Fillmore, Utah, while he was prospecting for uranium.[11]
While gem beryls are ordinarily found in pegmatites and certain metamorphic rocks, bixbite occurs in topaz-bearing rhyolites. It formed by crystallizing under low pressure and high temperature from a pneumatolitic phase along fractures or within near-surfacemiarolitic cavities of the rhyolite. Associated minerals include bixbyite, quartz, orthoclase, topaz,spessartine, pseudobrookite andhematite. The red color is thought to be from manganesesubstituting for aluminium in the beryl structure.
Aquamarine and maxixe
Aquamarine (from Lat. aqua marina, “water of the sea”) is a blue or turquoisevariety of beryl. It occurs at most localities which yield ordinary beryl, some of the finest coming from Russia. The gem-gravel placer deposits of Sri Lanka contain aquamarine. Clear yellow beryl, such as occurs in Brazil, is sometimes called aquamarine chrysolite. When corundum presents the bluish tint of typical aquamarine, it is often termed Oriental aquamarine. The deep blue version of aquamarine is called maxixe. Its color fades to white when exposed to sunlight or is subjected to heat treatment, though the color returns with irradiation.
The pale blue color of aquamarine is attributed to Fe2+. The Fe3+ ions produce golden-yellow color, and when both Fe2+ and Fe3+ are present, the color is a darker blue as in maxixe. Decoloration of maxixe by light or heat thus may be due to the charge transfer Fe3+ and Fe2+.[4][12][13][14] Dark-blue maxixe color can be produced in green, pink or yellow beryl by irradiating it with high-energy particles (gamma rays, neutrons or even X-rays).[15]
In the United States, aquamarines can be found at the summit of Mt. Antero in the Sawatch Range in central Colorado. In Wyoming, aquamarine has been discovered in the Big Horn mountains, near Powder River Pass. In Brazil, there are mines in the states ofMinas Gerais,Espírito Santo and Bahia. The Mines of Colombia, Zambia, Madagascar, Malawi, Tanzaniaand Kenya also produce aquamarine.
The biggest aquamarine ever mined was found at the city of Marambaia, Minas Gerais, Brazil, in 1910. It weighed over 110 kg, and its dimensions were 48.5 cm long and 42 cm in diameter.
Culture usage
Emerald
Emerald refers to green beryl, colored by trace amounts of chromium and sometimes vanadium.[4][16] The word “emerald” comes from Latinsmaragdus, its original source being a Semitic word izmargad or theSanskrit word,marakata, meaning “green”.[17] Most emeralds are highlyincluded, so their brittleness (resistance to breakage) is classified as generally poor.
Emeralds in antiquity were mined by the Egyptians and in Austria, as well as Swat in northernPakistan.[18] A rare type of emerald known as a trapiche emerald is occasionally found in the mines of Colombia. A trapiche emerald exhibits a “star” pattern; it has raylike spokes of dark carbon impurities that give the emerald a six-pointed radial pattern. It is named for thetrapiche, a grinding wheel used to process sugarcane in the region. Colombian emeralds are generally the most prized due to their transparency and fire. Some of the most rare emeralds come from three main emerald mining areas in Colombia: Muzo, Coscuez, and Chivor. Fine emeralds are also found in other countries, such as Zambia, Brazil, Zimbabwe, Madagascar,Pakistan, India,Afghanistan and Russia. In the US, emeralds can be found in Hiddenite, North Carolina. In 1998, emeralds were discovered in theYukon.
Emerald is a rare and valuable gemstone and, as such, it has provided the incentive for developing synthetic emeralds. Both hydrothermal[19] and flux-growth synthetics have been produced. The first commercially successful emerald synthesis process was that of Carroll Chatham. The other large producer of flux emeralds was Pierre Gilson Sr., which has been on the market since 1964. Gilson’s emeralds are usually grown on natural colorless beryl seeds which become coated on both sides. Growth occurs at the rate of 1 mm per month, a typical seven-month growth run producing emerald crystals of 7 mm of thickness.[20] The green color of emeralds is attributed to presense of Fe3+ and Fe2+ ions.[12] [13][14]
Goshenite

Crystal structure of beryl
Colorless beryl is called goshenite. The name originates from Goshen, Massachusetts where it was originally described. Since all these color varieties are caused by impurities and pure beryl is colorless, it might be tempting to assume that goshenite is the purest variety of beryl. However, there are several elements that can act as inhibitors to color in beryl and so this assumption may not always be true. The name goshenite has been said to be on its way to extinction and yet it is still commonly used in the gemstone markets. Goshenite is found to some extent in almost all beryl localities. In the past, goshenite was used for manufacturing eyeglasses and lenses owing to its transparency. Nowadays, it is most commonly used for gemstone purposes and also considered as a source of beryllium. [21][22]
The gem value of gohenite is relatively low. However, goshenite can be colored yellow, green, pink, blue and in intermediate colors by irradiating it with high-energy particles. The resoluting color depends on the content of Ca, Sc, Ti, V, Fe, and Co inmpurities.[12]
Golden beryl and heliodor
Golden beryl can range in colors from pale yellow to a brilliant gold. Unlike emerald, golden beryl has very few flaws. The term “golden beryl” is sometimes synonymous with heliodor(from Greek helios “sun”), but golden beryl refers to pure yellow or golden yellow shades, while heliodor refers to the greenish-yellow shades. The golden yellow color is attributed to Fe3+ ions.[4][12] Both golden beryl and heliodor are used as gems. Probably the largest cut golden beryl is the flawless 2054 carat stone on display on display in the Hall of Gems,Washington, D.C.[23]
Sapphire
Sapphire (Greek: sappheiros) refers to gem varieties of the mineral corundum, an aluminium oxide (α-Al2O3), when it is a color other than red, in which case the gem would instead be aruby. Trace amounts of other elements such as iron, titanium, or chromiumcan give corundum blue, yellow, pink, purple, orange, or greenish color. Pink-orange corundum are also sapphires, but are instead called padparadscha.
Because it is a gemstone, sapphire is commonly worn as jewelry. Sapphire can be found naturally, or manufactured in large crystalboules. Because of its remarkable hardness, sapphire is used in many applications, including infrared optical components, watchcrystals, high-durability windows, and wafers for the deposition of semiconductors.
| Sapphire |
 |
| General |
| Category |
Mineral Variety |
| Chemical formula |
aluminium oxide, Al2O3 |
| Identification |
| Color |
Every color except red (which is ruby) or pinkish-orange (padparadscha) |
| Crystal habit |
massive and granular |
| Crystal system |
Trigonal (Hexagonal Scalenohedral) Symbol (-3 2/m) Space Group: R-3c |
| Cleavage |
None |
| Fracture |
Conchoidal, splintery |
| Mohs Scalehardness |
9.0 |
| Luster |
Vitreous |
| Streak |
White |
| Specific gravity |
3.95–4.03 |
| Optical properties |
Abbe number 72.2 |
| Refractive index |
nω=1.768 – 1.772 nε=1.760 – 1.763,Birefringence 0.008 |
| Pleochroism |
Strong |
| Melting point |
2030–2050 °C |
| Fusibility |
infusible |
| Solubility |
Insoluble |
|
Natural sapphires
Sapphire is one of the two gem varieties of corundum, the other being the red ruby. Although blue is the most well known hue, sapphire is any color of corundum except red. Sapphire may also be colorless, and it also occurs in the non-spectral shades gray and black. Pinkish-orange sapphire is known aspadparadscha.
The cost of natural sapphire varies depending on their color, clarity, size, cut, and overall quality as well as geographic origin. Significant sapphire deposits are found in Eastern Australia, Thailand, Sri Lanka, Madagascar, East Africa and in the United States at various locations (Gem Mountain) and in the Missouri River near Helena, Montana. [1] Sapphire and rubies are often found together in the same area, but one gem is usually more abundant.[2]
Blue sapphire
Color in gemstones breaks down into three components:hue,saturation, and tone. Hue is most commonly understood as the “color” of the gemstone. Saturation refers to the vividness or brightness or “colorfulness” of the hue, and tone is the lightness to darkness of the hue.[3] Blue sapphire exists in various mixtures of its primary and secondary hues, various tonal levels (shades) and at various levels of saturation (brightness): the primary hue must, of course, be blue.
Blue sapphires are evaluated based upon the purity of their primary hue.Purple, violet and green are the normal secondary hues found in blue sapphires. [4] Violet and purple can contribute to the overall beauty of the color, while green is considered a distinct negative. [4] Blue sapphires with no more than 15% violet or purple are generally said to be of fine quality. [4] Blue sapphires with any amount of green as a secondary hue are not considered to be fine quality.[4] Gray is the normal saturation modifier or mask found in blue sapphires.[4] Gray reduces the saturation or brightness of the hue and therefore has a distinctly negative effect.
The color of fine blue sapphires can be described as a vivid medium dark violet to purplish blue where the primary blue hue is at least 85% and the secondary hue no more than 15% without the least admixture of a green secondary hue or a gray mask. [3]
The 422.99 carats (84.60 g) Logan sapphire in the National Museum of Natural History, Washington D.C. is one of the largest faceted gem-quality blue sapphires in the world.
Source of Color
Red rubies are corundum which contain chromium impurities that absorb yellow-green light and result in deeper ruby red color with increasing content.[5] Purple sapphires contain trace amounts ofvanadium and come in a variety of shades. Corundum that contains ~0.01% of titanium is colorless. If trace amounts of iron are present, a very pale yellow to green color may be seen. If both titanium and iron impurities are present together, however, the result is a magnificent deep-blue color.
Unlike localized (“interatomic”) absorption of light which causes color for chromium and vanadium impurities, blue color in sapphires comes from intervalence charge transfer, which is the transfer of an electron from one transition-metal ion to another via the conductionorvalence band. The iron can take the form Fe2+ or Fe3+, while titanium generally takes the form Ti4+. If Fe2+and Ti4+ ions are substituted for Al3+, localized areas of charge imbalance are created. An electron transfer from Fe2+ and Ti4+ can cause a change in the valence state of both. Because of the valence change there is a specific change in energy for the electron, andelectromagnetic energy is absorbed. The wavelength of the energy absorbed corresponds to yellow light. When this light is subtracted from incident white light, the complementary color blue results. Sometimes when atomic spacing is different in different directions there is resulting blue-green dichroism.
Intervalence charge transfer is a process that produces a strong colored appearance at a low percentage of impurity. While at least 1% chromium must be present in corundum before the deep red ruby color is seen, sapphire blue is apparent with the presence of only 0.01% of titanium and iron.
Fancy color sapphire
Purple sapphires are lower in price than blue ones. Yellow and green sapphires are also commonly found. Pink sapphires deepen in color as the quantity of chromium increases. The deeper the pink color the higher their monetary value as long as the color is going towards the red of rubies.
Sapphires also occur in shades of orange and brown, and colorless sapphires are sometimes used as diamond substitutes in jewelry. Salmon-colored padparadscha (see below) sapphires often fetch higher prices than many of even the finest blue sapphires. Recently, sapphires of this color have appeared on the market as a result of a new treatment method called “lattice diffusion”.[citation needed]
Padparadscha
Padparadscha is a pinkish-orange to orangy-pink colored corundum, with a low to medium saturation and light tone, originally being mined in Sri Lanka, but also found in deposits in Vietnam and Africa. Padparadscha sapphires are very rare, and highly valued for their subtle blend of soft pink and orange hues. The name derives from the Sinhalese word for lotus blossom. Along with rubiesthey are the only corundums to be given their own name instead of being called a particular colored sapphire.
The rarest of all padparadschas is the totally natural variety, with no beryllium or other treatment, and no heating.
Color change sapphire
A rare variety of sapphire, known as color change sapphire, exhibits different colors in different light. Color change sapphires are blue in outdoor light and purple underincandescent indoor light. Color changes may also be pink in daylight to greenish under fluorescent light. Some stones shift color well and others only partially, in that some stones go from blue to bluish purple. While color change sapphires come from a variety of locations, the gem gravels ofTanzania is the main source.
Certain synthetic color-change sapphires are sold as “lab” or “synthetic” alexandrite, which is accurately called an alexandrite simulant (also called alexandrium) since the latter is actually a type ofchrysoberyl—an entirely different substance whose pleochroism is different and much more pronounced than color-change corundum (sapphire).
Star sapphire

The 182 carats (36 g) Star of Bombay star sapphire
A star sapphire is a type of sapphire that exhibits a star-like phenomenon known as asterism. Star sapphires contain intersecting needle-like inclusions (often the mineral rutile, a mineral composed primarily oftitanium dioxide[6]) that cause the appearance of a six-rayed ‘star’-shaped pattern when viewed with a single overhead light source.
The value of a star sapphire depends not only on the carat weight of the stone but also the body color, visibility and intensity of the asterism.
The Star of India is thought to be the largest star sapphire in the world and is currently on display at theAmerican Museum of Natural Historyin New York City. The 182 carat (36.4 g) Star of Bombay, housed in theNational Museum of Natural History, Washington D.C., is a good example of a blue star sapphire..
Treatments
Sapphires may be treated by several methods to enhance and improve their clarity and color. [7] It is common practice to heat natural sapphires to improve or enhance color. This is done by heating the sapphires to temperatures between 500 and 1800 °C for several hours, or by heating in a nitrogen-deficient atmosphere oven for seven days or more. Low Tube heating is where the stone is placed in a ceramic pot over charcoal, in which a man blows air through a bamboo tube to the charcoal creating more heat. The stone becomes a more blue in color but loses some of the silk. When high heat temperatures are used, the stone loses all of the silk and becomes clear under magnification. Evidence of sapphire and other gemstones being subjected to heating goes back to, at least, Roman times. [8] Un-heated stones are quite rare and will often be sold accompanied by a certificate from an independent gemological laboratory attesting to “no evidence of heat treatment”.
Diffusion treatments are somewhat more controversial as they are used to add elements to the sapphire for the purpose of improving colors. Typically beryllium (Be) is diffused into a sapphire with very high heat, just below the melting point of the sapphire. Initially (c. 2000) orange sapphires were created with this process, although now the process has been advanced and many colors of sapphire are often treated with beryllium. It is unethical to sell beryllium-treated sapphires without disclosure, and the price should be much lower than a natural gem or one that has been enhanced by heat alone.
Treating stones with surface diffusion is generally frowned upon; as stones chip or are repolished/refaceted the ‘padparadscha’ colored layer can be removed. (There are some diffusion treated stones in which the color goes much deeper than the surface, however.) The problem lies in the fact that treated padparadschas are at times very difficult to detect, and they are the reason that getting a certificate from a reputable gemological lab (e.g. Gubelin, SSEF, AGTA, etc.) is recommended before investing in a padparadscha.
According to Federal Trade Commission guidelines, in the United States, disclosure is required of any mode of enhancement that has a significant effect on the gem’s value.[9]
Mining
Sapphires are mined fromalluvialdeposits or from primary underground workings.
The finest specimens were mined inKashmir, in the northwestern section of India, from about 1880 to 1920. They have also been mined inMyanmar, Madagascar, Sri Lanka,Australia, Thailand, India,Pakistan,Afghanistan, Tanzania,Kenya andChina. All three famous sapphires, the Logan sapphire, theStar of Indiaand the Star of Bombayoriginate from Sri Lankan mines. Madagascar leads the world in sapphire production (as of 2007) specifically in and around the city ofIlakaka.[citation needed] Prior to Ilakaka, Australia was the largest producer of sapphires (as of 1987).[citation needed] In the United States sapphires have been produced from deposits nearHelena, Montana. Gem grade sapphires and rubies are also found in and aroundFranklin, North Carolina..
In 1991 a new sapphire occurrence, similar in quality to that of Kashmir, was discovered in Andranondambo, southern Madagascar. That area was industrially exploited since 1993 and has been almost abandoned few years later because of difficulties of exploiting sapphires in their bedrock.[citation needed]
[edit] Synthetic sapphire
In 1902, French chemist Auguste Verneuil developed a process for growing synthetic sapphire crystals.[10] In the Verneuil process, fine alumina powder is added to anoxyhydrogen flame which is directed downward against a mantle.[11]Alumina in the flame is slowly deposited, creating a teardrop shaped ‘boule‘ of sapphire. Chemical dopants can be added to create artificial versions of ruby and all the other sapphire gems, plus colors never seen in nature. Artificial sapphire is identical to natural sapphire, except it can be made without the flaws found in natural stones. However the Verneuil process had the disadvantage that the crystals created with it had high internal strains. Many methods of manufacturing sapphire today are variations of the Czochralski process, invented in 1916.[12] A tiny sapphire seed crystal is dipped into a crucible of molten alumina and slowly withdrawn upward at a rate of 1 to 100 mm per hour. The alumina crystallizes on the end, creating long carrot shaped boules of large size, up to 400 mm in diameter and weighing almost 500 kg.[13]
In 2003, the world’s production of synthetic sapphire was 250 tons.(1.25 x 109carats).[13] The availability of cheap synthetic sapphire unlocked many industrial uses for this unique material:
The first laser was made with a rod of synthetic ruby. Titanium-sapphire lasers are popular due to the relatively rare ability to tune the laser wavelength in the red-to nearinfraredregion of theelectromagnetic spectrum.. They can also be easilymode-locked. In these lasers, a synthetically produced sapphirecrystal with chromium ortitaniumimpurities is irradiated with intense light from a special lamp, or another laser, to create stimulated emission.
One application of synthetic sapphire is sapphire glass. Sapphire is not only highly transparent to wavelengths of light between 170 nm to 5.3 μm (the human eye can discern wavelengths from around 400 nm to 700 nm), but it is also five times stronger than glass and ranks a 9 on the Mohs Scale, although it is also more brittle. Sapphire glass is made from pure sapphire boules by slicing off and polishing thin wafers. Sapphire glass windows are used in high pressure chambers forspectroscopy, crystals in high qualitywatches, and windows in grocery store barcode scannerssince the material’s exceptional hardness makes it very resistant to scratching.[13] Owners of such watches should still be careful to avoid exposure to diamond jewelry, and should avoid striking their watches against artificial stone and simulated stone surfaces that often contain silicon carbide and other materials that are harder than sapphire and thus capable of causing scratches.

Cermax xenon arc lamp with synthetic sapphire output window
One type of xenon arc lamp, known as Cermax (original brand name — generically known as a ceramic body xenon lamp), uses sapphire output windows that are doped with various other elements to tune their emission. In some cases, the UV emitted from the lamp during operation causes a blue glow from the window after the lamp is turned off. It is approximately the same color as Cherenkov radiation but is caused by simplephosphorescence.
Wafers of single-crystal sapphire are also used in thesemiconductorindustry as asubstrate for the growth of devices based on gallium nitride(GaN), with a transparent conductive coating (TCC) formed from gallium nitride on a sapphire substrate. In order to account for the lattice mismatch between the GaN and the sapphire substrate, a nucleation layer is formed on the sapphire substrate. A mask, for examplesilicon dioxide(SiO2), is formed on top of the nucleation layer with a plurality of openings. GaN is then grown through the openings in the mask to form a lateral epitaxial overgrowth layer upon which defect-free GaN is then grown. The lateral epitaxial overgrowth compensates for the lattice mismatch between the sapphire substrate and the GaN. The use of a sapphire substrate eliminates the need for a cover glass and also significantly reduces the cost of the TCC, since such sapphire substrates are about one-seventh the cost of germanium substrates. Gallium nitride on sapphire is commonly used in blue light-emitting diodes (LEDs).
The transparent conductive coating (TCC) may then be disposed on agallium arsenide (GaAs) solar cell. In order to compensate for the lattice mismatches between the GaAs and the GaN, an indium gallium phosphate (InGaP) may be disposed between the GaAs solar cell and the GaN TCC to compensate for the lattice mismatch between the GaN and the GaAs. In order to further compensate for the lattice mismatch between the GaN and InGaP, the interface may be formed as a super lattice or as a graded layer. Alternatively, the interface between the GaN and the InGaP may be formed by the offset method or by wafer fusion. The TCC, in accordance with the present invention, is able to compensate for the lattice mismatches at the interfaces of the TCC while eliminating the need for a cover glass and a relatively expensive germanium substrate.
Historical and cultural references
- According to Rebbenu Bachya, and many English Bible translations, the wordSapir in the verseExodus28:18 meanssapphire and was the stone on the Ephodrepresenting the tribe ofIssachar. Although it has been stated that the English word sapphirederives from the Hebrewsapir (via Greeksapphiros), this is disputed. Sapphires were actually not known before the Roman Empire (and were initially considered to be forms ofjacinth, rather than deserving of a word to themselves), and prior to that time sapphirosreferred to blue gems in general.
- Sapphire is thebirthstoneassociated with September.
- The 45th wedding anniversary is known as the sapphire anniversary.
|
Ruby

Crystal structure of ruby
A ruby is a pink to blood-red gemstone, a variety of the mineral corundum (aluminium oxide). The red color is caused mainly by the presence of the element chromium. Its name comes from ruber, Latin for red. Other varieties of gem-quality corundum are calledsapphires. The ruby is considered one of the four precious stones, together with the sapphire, the emerald, and thediamond.[citation needed]
Prices of rubies are primarily determined by color. The brightest and most valuable “red” called pigeon blood-red, commands a huge premium over other rubies of similar quality. After color follows clarity: similar to diamonds, a clear stone will command a premium, but a ruby without any needle-like rutile inclusions may indicate that the stone has been treated. Cut and carat(size) also determine the price.
Physical properties
Rubies have a hardness of 9.0 on the Mohs scale of mineral hardness. Among the natural gems only moissanite and diamond are harder, with diamond having a Mohs hardness of 10.0 and moissonite falling somewhere in between corundum (ruby) and diamond in hardness. Ruby is α-alumina (the most stable form of Al2O3) in which a small fraction of the aluminum3+ions are replaced by chromium3+ ions. Each Cr3+ is surrounded octahedrally by six O2- ions. This crystallographic arrangement strongly affects each Cr3+, resulting in light absorption in the yellow-green region of the spectrum and thus in the red color of the gem. When yellow-green light is absorbed by Cr3+, it is re-emitted as red luminescence.[1] This red emission adds to the red colour perceived by the subtraction of green and violet light from white light, and adds luster to the gem’s appearance. When the optical arrangement is such that the emission is stimulated by 694-nanometer photons reflecting back and forth between two mirrors, the emission grows strongly in intensity. This effect was used by Theodore Maiman in 1960 to make the first successful laser, based on ruby.
All natural rubies have imperfections in them, including color impurities and inclusions of rutileneedles known as “silk”. Gemologists use these needle inclusions found in natural rubies to distinguish them from synthetics, simulants, or substitutes. Usually the rough stone is heated before cutting. Almost all rubies today are treated in some form, with heat treatment being the most common practice. However, rubies that are completely untreated but still of excellent quality command a large premium.
Some rubies show a 3-point or 6-point asterism or “star”. These rubies are cut into cabochonsto display the effect properly. Asterisms are best visible with a single-light source, and move across the stone as the light moves or the stone is rotated. Such effects occur when light is reflected off the “silk” (the structurally oriented rutile needle inclusions) in a certain way. This is one example where inclusions increase the value of a gemstone. Furthermore, rubies can show color changes — though this occurs very rarely —; as well as chatoyancy or the “cat’s eye” effect.
Natural occurrence
Rubies have historically been mined in Thailand, the Pailin and Samlot provinces of Cambodia, and Afghanistan. Rubies were rarely found in Sri Lanka where pink sapphires are more common.
After the Second World War new ruby deposits were found in Tanzania, Kenya, Madagascar,Vietnam, Nepal, Tajikistan, andPakistan. They have also been sometimes found in the U.S. states of Montana, North Carolina, and South Carolina. More recently,large ruby depositshave been found under the receding ice shelf of Greenland. The Mogok Valley in Upper Myanmar was for centuries the world main source for rubies. It has produced some of the finest rubies ever mined, but in recent years very few good rubies have been found there. The very best color in Myanmar (Burmese) rubies is sometimes described as “pigeon’s blood”. In central Myanmar the area of Mong Hsu also started to produce rubies during the 1990s and rapidly became the world’s main ruby mining area. The latest ruby deposit to be found in Myanmar is situated in Namya (Namyazeik) located in the northern Kachin state. In 2002 rubies were found in the Waseges River area of Kenya. Spinel, another red gemstone, is sometimes found associated with rubies from the same gem gravel or marble. Red spinel may be mistaken with ruby by people lacking experience with gems. However, fine red spinels may approach the average ruby in value.[2]
Factors affecting value
Diamonds are graded using criteria that have become known as the four Cs, namely color, cut, clarity and carat weight. Similarly natural rubies can be evaluated using the four Cs together with their size and geographic origin.
Color: In the evaluation of colored gemstones, color is the single most important factor. Color divides into three components; hue,saturation and tone. Hue refers to “color” as we normally use the term. Transparent gemstones occur in the following hues: red, orange, yellow, green, blue, violet, purple and pink are the spectral hues. The first six are known as spectral hues; the last two are modified spectral hues. Purple is a hue that falls halfway between red and blue and pink is a paler shade of red.[3] In nature there are rarely pure hues so when speaking of the hue of a gemstone we speak of primary and secondary and sometimes tertiary hues. In ruby the primary hue must be red. All other hues of the gem species corundum are called sapphire. Ruby may exhibit a range of secondary hues. Orange, purple, violet and pink are possible.
Natural Ruby with inclusions
|
|
The finest ruby is best described as being a vivid medium-dark toned red. Secondary hues add an additional complication. Pink, orange, and purple are the normal secondary hues in ruby. Of the three, purple is preferred because, firstly, the purple reinforces the red making it appear richer[4]. Secondly, purple occupies a position on the color wheel halfway between red and blue. In Burma where the term pigeon blood originated, rubies are set in pure gold. Pure gold is, itself a highly saturated yellow. Set a purplish-red ruby in yellow and the yellow neutralizes its compliment blue leaving the stone appearing to be pure red in the setting[5].
Treatments and enhancements
Improving the quality of gemstones by treating them is common practice. Some treatments are used in almost all cases and are therefore considered acceptable. During the late 1990s, a large supply of low-cost materials caused a sudden surge in supply of heat-treated rubies, leading to a downward pressure on ruby prices.
Improvements used include color alteration, improving transparency by dissolving rutile inclusions, healing of fractures (cracks) or even completely filling them.
The most common treatment is the application of heat. Most, if not all, rubies at the lower end of the market are heat treated on the rough stones to improve color, remove purple tinge, blue patches and silk. These heat treatments typically occur around temperatures of 1800 °C (3300 °F).[6] Some rubies undergo a process of low tube heat, when the stone is heated over charcoal of a temperature of about 1300 °C (2400 °F) for 20 to 30 minutes. The silk is only partially broken as the color is improved.
A less acceptable treatment, which has gained notoriety in recent years, is lead glass filling. Filling the fractures inside the ruby withlead glass dramatically improves the transparency of the stone, making previously unsuitable rubies fit for applications in jewelry. The process is done in four steps:
- The rough stones are pre-polished to eradicate all surface impurities that may affect the process
- The rough is cleaned with hydrogen fluoride
- The first heating process during which no fillers are added. The heating process eradicates impurities inside the fractures. Although this can be done at temperatures up to 1400 °C (2500 °F) it most likely occurs at a temperature of around 900 °C (1600 °F) since the rutile silk is still intact
- The second heating process in an electrical oven with different chemical additives. Different solutions and mixes have shown to be successful, however mostly lead-containing glass-powder is used at present. The ruby is dipped into oils, then covered with powder, embedded on a tile and placed in the oven where it is heated at around 900 °C (1600 °F) for one hour in an oxidizing atmosphere. The orange colored powder transforms upon heating into a transparent to yellow-colored paste, which fills all fractures. After cooling the color of the paste is fully transparent and dramatically improves the overall transparency of the ruby.
If a color needs to be added, the glass powder can be “enhanced” with copper or other metal oxides as well as elements such as sodium, calcium, potassium etc.
The second heating process can be repeated three to four times, even applying different mixtures.[7] When jewelry containing rubies is heated (for repairs) it should not be coated with boracic acid or any other substance, as this can etch the surface; it does not have to be “protected” like a diamond.
Synthetic and imitation rubies
In 1837 Gaudin made the first synthetic rubies by fusing aluminium at a high temperature with a little chromium as a pigment. In 1847 Ebelmen made white sapphire by fusing alumina in boric acid. In 1877 Frenic and Freil made crystal corundum from which small stones could be cut. Frimy and Auguste Verneuil manufactured artificial ruby by fusing BaF2 and Al2O3 with a little Chromium at red heat. In 1903 Verneuil announced he could produce synthetic rubies on a commercial scale using this flame fusion process.[8]
Other processes in which synthetic rubies can be produced are through the Pulling process, flux process, and the hydrothermal process. Most synthetic rubies originate from flame fusion, due to the low costs involved. Synthetic rubies may have no imperfections visible to the naked eye but magnification may reveal curves striae and gas bubbles. The fewer the number and the less obvious the imperfections, the more valuable the ruby is; unless there are no imperfections (i.e., a “perfect” ruby), in which case it will be suspected of being artificial.Dopants are added to some manufactured rubies so they can be identified as synthetic, but most need gemmological testing to determine their origin.
Synthetic rubies have technological uses as well as gemological ones. Rods of synthetic ruby are used to make ruby lasers andmasers. The first working laser was made by Theodore H. Maiman in 1960[9] at Hughes Research Laboratories in Malibu, California, beating several research teams including those of Charles H. Townes at Columbia University, Arthur Schawlow at Bell Labs,[10] and Gould at a company called TRG (Technical Research Group). Maiman used a solid-state light-pumped synthetic ruby to produce red laser light at a wavelength of 694 nanometers (nm). Ruby lasers are still in use.
Imitation rubies are also marketed. Red spinels, red garnets, and colored glass have been falsely claimed to be rubies. Imitations go back to Roman times and already in the 17th century techniques were developed to color foil red—by burning scarlet wool in the bottom part of the furnace—which was then placed under the imitation stone.[11] Trade terms such asbalas ruby for red spinel andrubellite for red tourmaline can mislead unsuspecting buyers. Such terms are therefore discouraged from use by many gemological associations such as the Laboratory Manual Harmonisation Committee (LMHC).
Records

Rubies at the National Museum of Natural History, Washington DC, USA
The Smithsonian’s National Museum of Natural History in Washington DC, has received one of the world’s largest and finest ruby gemstones. The 23.1 carats (4.6 g) Burmese ruby, set in a platinum ring with diamonds, was donated by businessman and philanthropist Peter Buck in memory of his wife Carmen Lúcia. This gemstone displays a richly saturated red color combined with an exceptional transparency. The finely proportioned cut provides vivid red reflections. The stone was mined from the famous Mogokregion of Burma (now Myanmar) in the 1930s.[12]
Synthetic Ruby is also used extensively in the metrology field, serving as stylii material for contact measuring instruments such as a Coordinate Measuring Machine, (CMM).
Historical and cultural references
- An early recorded note of the transport and trading of rubies arises in the literature on the North Silk Road of China, where in about 200 BC rubies were carried along this ancient trackway moving westward from China.[13]
- Rubies have always been held in high esteem in Asian countries. They were used to ornament armor, scabbards, and harnesses of noblemen in India and China. Rubies were laid beneath the foundation of buildings to secure good fortune to the structure.
|
Turquoise
Turquoise is an opaque, blue-to-green mineral that is a hydrous phosphate of copper andaluminium, with the chemical formulaCuAl6(PO4)4(OH)8·4H2O. It is rare and valuable in finer grades and has been prized as a gem and ornamental stone for thousands of years owing to its unique hue. In recent times turquoise, like most other opaque gems, has been devalued by the introduction of treatments, imitations, and synthetics onto the market.
The substance has been known by many names, but the word turquoise was derived around the 16th century from the French language either from the word for Turkish (Turquois) or dark-blue stone (pierre turquin).[4] This may have arisen from a misconception: turquoise does not occur in Turkey but was traded at Turkish bazaars to Venetian merchants who brought it to Europe.[4] In addition, the colour has been employed extensively in the decorative tiles adorning Turkish places of worship and homes at least since the 14th century. Another conjecture is that the name refers to the colour of the Mediterranean Sea on the southern Turkish coast.
Properties of turquoise
Even the finest of turquoise is fracturable, reaching a maximum hardness of just under 6, or slightly more than window glass.[2]Characteristically a cryptocrystalline mineral, turquoise almost never forms single crystals and all of its properties are highly variable. Its crystal system is proven to be triclinic via X-ray diffraction testing. With lower hardness comes lowerspecific gravity (2.60–2.90) and greater porosity: These properties are dependent on grain size. The lustre of turquoise is typically waxy to subvitreous, andtransparency is usually opaque, but may be semitranslucent in thin sections. Colour is as variable as the mineral’s other properties, ranging from white to a powder blue to a sky blue, and from a blue-green to a yellowish green. The blue is attributed to idiochromaticcopper while the green may be the result of either iron impurities (replacing aluminium) or dehydration.
The refractive index (as measured by sodium light, 589.3 nm) of turquoise is approximately 1.61 or 1.62; this is a mean value seen as a single reading on a gemmological refractometer, owing to the almost invariably polycrystalline nature of turquoise. A reading of 1.61–1.65 (birefringence 0.040, biaxial positive) has been taken from rare single crystals. An absorption spectrum may also be obtained with a hand-held spectroscope, revealing a line at 432 nanometres and a weak band at 460 nanometres (this is best seen with strong reflected light). Under longwave ultraviolet light, turquoise may occasionally fluoresce green, yellow or bright blue; it is inert under shortwave ultraviolet and X-rays.
Turquoise is insoluble in all but heated hydrochloric acid. Its streak is a pale bluish white and its fracture is conchoidal, leaving a waxy lustre. Despite its low hardness relative to other gems, turquoise takes a good polish. Turquoise may also be peppered with flecks of pyrite or interspersed with dark, spidery limonite veining.
Formation
As a secondary mineral, turquoise apparently forms by the action of percolating acidic aqueous solutions during the weathering andoxidation of pre-existing minerals. For example, the copper may come from primary copper sulfides such as chalcopyrite or from the secondary carbonates malachite or azurite; the aluminium may derive from feldspar; and the phosphorus from apatite. Climate factors appear to play an important role as turquoise is typically found in arid regions, filling or encrusting cavities and fractures in typically highly altered volcanic rocks, often with associated limonite and other iron oxides. In the American southwest turquoise is almost invariably associated with the weathering products of copper sulfide deposits in or around potassium feldspar bearing porphyritic intrusives. In some occurrences alunite, potassium aluminium sulfate, is a prominent secondary mineral. Typically turquoise mineralization is restricted to a relatively shallow depth of less than 20 metres (66 ft), although it does occur along deeper fracture zones where secondary solutions have greater penetration or the depth to the water table is greater.
Although the features of turquoise occurrences are consistent with a secondary or supergeneorigin, some sources refer to ahypogene origin. The hypogene hypothesis, which holds that the aqueous solutions originate at significant depth, from hydrothermalprocesses. Initially at high temperature, these solutions rise upward to surface layers, interacting with and leaching essential elements from pre-existing minerals in the process. As the solutions cool, turquoise precipitates, lining cavities and fractures within the surrounding rock. This hypogene process is applicable to the original copper sulfide deposition; however, it is difficult to account for the many features of turquoise occurrences by a hypogene process. That said, there are reports of two phase fluid inclusionswithin turquoise grains that give elevated homogenization temperatures of 90 to 190 °C that require explanation.
Turquoise is nearly always cryptocrystalline and massive and assumes no definite external shape. Crystals, even at the microscopic scale, are exceedingly rare. Typically the form is vein or fracture filling, nodular, or botryoidal in habit. Stalactite forms have been reported. Turquoise may also pseudomorphously replace feldspar, apatite, other minerals, or evenfossils. Odontolite is fossil bone orivory that has been traditionally thought to have been altered by turquoise or similar phosphate minerals such as the iron phosphatevivianite. Intergrowth with other secondary copper minerals such as chrysocolla is also common.
Occurrence
Turquoise was among the first gems to be mined, and while many historic sites have been depleted, some are still worked to this day. These are all small-scale, often seasonal operations, owing to the limited scope and remoteness of the deposits. Most are worked by hand with little or no mechanization. However, turquoise is often recovered as a byproduct of large-scale copper mining operations, especially in the United States.
Iran

Cutting and grinding turquoise in Meshed, Iran. 1973.
For at least 2,000 years, the region once known as Persia, has remained the most important source of turquoise, for it is here that fine material is most consistently recovered. This “perfect colour” deposit, which is blue naturally, and turns green when heated due to dehyration, is restricted to a mine-riddled region in Neyshabur,[5][6][7] the 2,012-metre (6,600 ft) mountain peak of Ali-mersai, which is tens of kilometers from Mashhad, the capital of Khorasan province, Iran. A weathered and broken trachyte is host to the turquoise, which is found both in situ between layers of limonite and sandstone, and amongst the scree at the mountain’s base. These workings, together with those of the Sinai Peninsula, are the oldest known.
Iranian turquoise is often found replacing feldspar. Although it is commonly marred by whitish patches, its colour and hardness are considered superior to the production of other localities. Iranian turquoise has been mined and traded abroad for centuries, and was probably the source of the first material to reach Europe.
Sinai
Since at least the First Dynasty (3000 BCE), and possibly before then, turquoise was used by the Egyptians and was mined by them in the Sinai Peninsula, called “Country of Turquoise” by the native Monitu. There are six mines in the region, all on the southwest coast of the peninsula, covering an area of some 650 square kilometres (250 sq mi). The two most important of these mines, from a historic perspective, are Serabit el-Khadim and Wadi Maghareh, believed to be among the oldest of known mines. The former mine is situated about 4 kilometres from an ancient temple dedicated to Hathor.
The turquoise is found in sandstone that is, or was originally, overlain by basalt. Copper and iron workings are present in the area. Large-scale turquoise mining is not profitable today, but the deposits are sporadically quarried by Bedouin peoples using homemadegunpowder. In the rainy winter months, miners face a risk from flash flooding; even in the dry season, death from the collapse of the haphazardly exploited sandstone mine walls is not unheard of. The colour of Sinai material is typically greener than Iranian material, but is thought to be stable and fairly durable. Often referred to as Egyptian turquoise, Sinai material is typically the most translucent, and under magnification its surface structure is revealed to be peppered with dark blue discs not seen in material from other localities.
In proximity to nearby Eilat, Israel, an attractive intergrowth of turquoise, malachite, and chrysocolla is found. This rock is called Eilat stone and is often referred to as Israel’s national stone: it is worked by local artisans for sale to tourists.
United States

Bisbee turquoise commonly has a hard chocolate brown coloured matrix.
The Southwest United States is a significant source of turquoise; Arizona, California (San Bernardino, Imperial, and Inyo counties),Colorado (Conejos, El Paso, Lake, and Saguachecounties), New Mexico (Eddy, Grant, Otero, and Santa Fe counties) and Nevada(Clark, Elko,Esmerelda County, Eureka, Lander, Mineral County and Nye counties) are (or were) especially rich. The deposits of California and New Mexico were mined by pre-ColumbianNative Americans using stone tools, some local and some from as far away as centralMexico. Cerrillos, New Mexico is thought to be the location of the oldest mines; prior to the 1920s, the state was the country’s largest producer; it is more or less exhausted today. Only one mine in California, located at Apache Canyon, operates at a commercial capacity today.
The turquoise occurs as vein or seam fillings, and as compact nuggets; these are mostly small in size. While quite fine material—rivalling Iranian material in both colour and durability—is sometimes found, most American turquoise is of a low grade (called “chalk turquoise”); high iron levels mean greens and yellows predominate, and a typically friable consistency precludes use in jewellery in the turquoise’s untreated state. Arizona is currently the most important producer of turquoise by value.[citation needed] Two mines exist in the state, one is the Sleeping Beauty Mine in Globe, the other is the Kingman Mine that operates alongside a copper mine outside of the city.
Nevada is the country’s other major producer, with more than 120 mines which have yielded significant quantities of turquoise. Unlike elsewhere in the US, most Nevada mines have been worked primarily for their gem turquoise and very little has been recovered as a byproduct of other mining operations. Nevada turquoise is found as nuggets, fracture fillings and in breccias as the cement filling interstices between fragments. Because of the geology of the Nevada deposits, a majority of the material produced is hard and dense, being of sufficient quality that no treatment or enhancement is required.. While nearly every county in the state has yielded some turquoise, the chief producers are in Lander and Esmerelda Counties. Most of the turquoise deposits in Nevada occur along a wide belt of tectonic activity that coincides with the state’s zone of thrust faulting. It strikes about N15E and extends from the northern part of Elko County, southward down to the California border southwest of Tonopah. Nevada has produced a wide diversity of colours and mixes of different matrix patterns, with turquoise from Nevada coming in various shades of blue, blue-green, and green. Nevada produces some unique shades of bright mint to apple to neon yellow green. Some of this unusually coloured turquoise may contain significant zinc and iron, which is the cause of the beautiful bright green to yellow-green shades. Some of the green to green yellow shades may actually be Varisciteor Faustite, which are secondary phosphate minerals similar in appearance to turquoise. A significant portion of the Nevada material is also noted for its often attractive brown or black limonite veining, producing what is called “spiderweb matrix”. While a number of the Nevada deposits were first worked by Native Americans, the total Nevada turquoise production since the 1870s has been estimated at more than 600 tons, including nearly 400 tons from the Carico Lake mine. In spite of increased costs, small scale mining operations continue at a number of turquoise properties in Nevada, including the Godber, Orvil Jack and Carico Lake Mines in Lander County, the Pilot Mountain Mine in Mineral County, and several properties in the Royston and Candelaria areas of Esmerelda County.[8]

Untreated turquoise, Nevada USA. Rough nuggets from the McGinness Mine, Austin; Blue and green cabochons showing spiderweb, Bunker Hill Mine, Royston
In 1912, the first deposit of distinct, single-crystal turquoise was discovered in Lynch Station,Campbell County, Virginia. The crystals, forming a druse over the mother rock, are very small; 1 mm (0.04 in) is considered large. Until the 1980s Virginia was widely thought to be the only source of distinct crystals; there are now at least 27 other localities.[9]
In an attempt to recoup profits and meet demand, some American turquoise is treated orenhanced to a certain degree. These treatments include innocuous waxing and more controversial procedures, such as dyeing and impregnation (see Treatments). There are however, some American mines which produce materials of high enough quality that no treatment or alterations are required. Any such treatments which have been performed should be disclosed to the buyer on sale of the material.
Other sources
China has been a minor source of turquoise for 3,000 years or more. Gem-quality material, in the form of compact nodules, is found in the fractured, silicified limestone of Yunxian andZhushan, Hubei province. Additionally, Marco Polo reported turquoise found in present-daySichuan. Most Chinese material is exported, but a few carvings worked in a manner similar tojade exist. In Tibet, gem-quality deposits purportedly exist in the mountains of Derge andNagari-Khorsum in the east and west of the region respectively.[10]
Other notable localities include: Afghanistan; Australia (Victoria and Queensland); northernChile (Chuquicamata); Cornwall; Saxony;Silesia; and Turkestan.
History of its use

Moche turquoise nose ornament..Larco MuseumCollection. Lima-Peru

Trade in turquoise crafts, such as this freeform pendant dating from 1000–1040CE, is believed to have brought the Ancestral Puebloans of the Chaco Canyon great wealth.
The pastel shades of turquoise have endeared it to many great cultures of antiquity: it has adorned the rulers of Ancient Egypt, theAztecs (and possibly other Pre-ColumbianMesoamericans), Persia, Mesopotamia, the Indus Valley, and to some extent in ancientChina since at least the Shang Dynasty.[11] Despite being one of the oldest gems, probably first introduced to Europe(through Turkey) with other Silk Road novelties, turquoise did not become important as an ornamental stone in the West until the 14th century, following a decline in the Roman Catholic Church‘s influence which allowed the use of turquoise in secular jewellery. It was apparently unknown in India until the Mughal period, and unknown in Japanuntil the 18th century.. A common belief shared by many of these civilizations held that turquoise possessed certain prophylactic qualities; it was thought to change colour with the wearer’s health and protect him or her from untoward forces.
The Aztecs inlaid turquoise, together with gold, quartz, malachite, jet, jade, coral, and shells, into provocative (and presumably ceremonial) mosaic objects such as masks (some with a human skull as their base), knives, and shields. Natural resins, bitumenand wax were used to bond the turquoise to the objects’ base material; this was usually wood, but bone and shell were also used. Like the Aztecs, the Pueblo, Navajo and Apache tribes cherished turquoise for its amuletic use; the latter tribe believe the stone to afford the archer dead aim. Among these peoples turquoise was used in mosaic inlay, in sculptural works, and was fashioned into toroidal beads and freeform pendants. The Ancestral Puebloans (Anasazi) of the Chaco Canyon and surrounding region are believed to have prospered greatly from their production and trading of turquoise objects. The distinctive silver jewelry produced by the Navajo and other Southwestern Native American tribes today is a rather modern development, thought to date from circa 1880 as a result of European influences.
In Persia, turquoise was the de facto national stone for millennia, extensively used to decorate objects (from turbans to bridles),mosques, and other important buildings both inside and out, such as the Medresseh-I Shah Husein Mosque of Isfahan. The Persian style and use of turquoise was later brought to India following the establishment of the Mughal Empire there, its influence seen in high purity gold jewellery (together with ruby and diamond) and in such buildings as the Taj Mahal. Persian turquoise was oftenengraved with devotional words inArabic script which was then inlaid with gold.
Cabochons of imported turquoise, along with coral, was (and still is) used extensively in the silver and gold jewellery of Tibet andMongolia, where a greener hue is said to be preferred. Most of the pieces made today, with turquoise usually roughly polished into irregular cabochons set simply in silver, are meant for inexpensive export to Western markets and are probably not accurate representations of the original style.
The Egyptian use of turquoise stretches back as far as the First Dynasty and possibly earlier; however, probably the most well-known pieces incorporating the gem are those recovered fromTutankhamun‘s tomb, most notably the Pharaoh‘s iconic burial mask which was liberally inlaid with the stone. It also adorned rings and great sweeping necklaces called pectorals. Set in gold, the gem was fashioned into beads, used as inlay, and often carved in a scarab motif, accompanied by carnelian, lapis lazuli, and in later pieces, coloured glass. Turquoise, associated with the goddess Hathor, was so liked by the Ancient Egyptians that it became (arguably) the first gemstone to be imitated, the fair structure created by an artificial glazedceramic product known as faience. (A similar blue ceramic has been recovered from Bronze Age burial sites in the British Isles.)
The French conducted archaeological excavations of Egypt from the mid-19th century through the early 20th. These excavations, including that of Tutankhamun’s tomb, created great public interest in the western world, subsequently influencing jewellery,architecture, and art of the time. Turquoise, already favoured for its pastel shades since c. 1810, was a staple of Egyptian Revivalpieces. In contemporary Western use, turquoise is most often encountered cut en cabochon in silver rings, bracelets, often in the Native American style, or as tumbled or roughly hewn beads in chunky necklaces. Lesser material may be carved into fetishes, such as those crafted by the Zuni. While strong sky blues remain superior in value, mottled green and yellowish material is popular withartisans. In Western culture, turquoise is also the traditional birthstone for those born in the month of December.
In Judeo-Christian scripture
Turquoise may have significance in Judeo-Christian scripture: In the Book of Exodus, the construction of a “breastplate of judgment” is described as part of the priestly vestments ofAaron (Exodus 28:15–30). Attached to the ephod, the breastplate (Hoshen) was adorned with twelve gemstones set in gold and arranged in four rows, each stone engraved with the name of one of the Twelve Tribes of Israel. Of the four stones in the third row, the first and second have been translated to be turquoise by various scholars and English bible versions (usually not having both as turquoise at the same time); many others disagree, however.[12]
In regard to the first of these stones, the translation is based on the Septuagint rendering the identity of the stone as chrysolithos(the masoretic text calls it tarshish, which just refers toTarshish, a place, and gives no clue to the gem in question); at the time it was writtenchrysolithos did not mean Chrysolite specifically, but only golden stone (chryso-lithos).Chrysolithos is considered by scholars to possibly mean Topaz, Chrysolite, yellow Jasper, yellow Serpentine, or Turquoise – the last of these on the basis that Turquoise contains golden flecks, and that targums identified the stone as being sea coloured. Scholars favour stones which are mostly yellow as being the more likely solution, and opaque stones (Jasper or Serpentine) as more likely than translucent ones, on the consideration of nearby stones in the Hoshen.
In regard to the second of these stones, the masoretic text calls it shoham, and the Septuagint calls it Beryllios (Beryl), though elsewhere it translates shoham as onychion(Onyx), or as smaragdos (green stone). Shoham is of uncertain meaning. Following the Septuagint, some people think the stone should be an onyx (and many more traditional English versions of the Bible take this translation), but scholars think that the stone is actuallyMalachite (because it is green like beryl and smaragdos, cloudy as beryl can be, and in bands like onyx).
Scholars also disagree as to which tribes of the Israelites each stone is meant to represent; traditional sources are in just as much disagreement.
Imitations
The Egyptians were the first to produce an artificial imitation of turquoise, in the glazed earthenware product faience. Later glass andenamel were also used, and in modern times more sophisticated ceramics, porcelain, plastics, and various assembled, pressed, bonded, and sintered products (composed of various copper and aluminium compounds) have been developed: examples of the latter include “Viennese turquoise”, made from precipitatedaluminium phosphate coloured by copper oleate; and “neolith”, a mixture ofbayerite andcopper phosphate. Most of these products differ markedly from natural turquoise in both physical and chemical properties, but in 1972 Pierre Gilson introduced one fairly close to a true synthetic (it does differ in chemical composition owing to a binder used, meaning it is best described as a simulant rather than a synthetic). Gilson turquoise is made in both a uniform colour and with black “spiderweb matrix” veining not unlike the natural Nevada material.

Some natural blue to blue-green materials, such as this botryoidal chrysocolla with quartz drusy, are occasionally confused with, or used to imitate turquoise.
The most common imitation of turquoise encountered today is dyed howlite and magnesite, both white in their natural states, and the former also having natural (and convincing) black veining similar to that of turquoise. Dyed chalcedony, jasper, and marble is less common, and much less convincing. Other natural materials occasionally confused with or used in lieu of turquoise include: varisciteand faustite;[13] chrysocolla (especially when impregnating quartz);lazulite; smithsonite; hemimorphite; wardite; and a fossil bone ortooth called odontolite or “bone turquoise”, coloured blue naturally by the mineral vivianite. While rarely encountered today, odontolite was once mined in large quantities—specifically for its use as a substitute for turquoise—in southern France.
These fakes are detected by gemmologists using a number of tests, relying primarily on non-destructive, close examination of surface structure under magnification; a featureless, pale blue background peppered by flecks or spots of whitish material is the typical surface appearance of natural turquoise, while manufactured imitations will appear radically different in both colour (usually a uniform dark blue) and texture (usually granular or sugary). Glass and plastic will have a much greater translucency, with bubbles or flow lines often visible just below the surface. Staining between grain boundaries may be visible in dyed imitations.
Some destructive tests may, however, be necessary; for example, the application of diluted hydrochloric acid will cause thecarbonates odontolite and magnesite to effervesce and howlite to turn green, while a heated probe may give rise to the pungent smell so indicative of plastic. Differences in specific gravity, refractive index, light absorption (as evident in a material’sabsorption spectrum), and other physical and optical properties are also considered as means of separation. Imitation turquoise is so prevalent that it likely outnumbers real turquoise by a wide margin. Even material used in authentic Native American and Tibetan jewellery is often fake or, at best, heavily treated[citation needed].
Treatments

An early turquoise mine in the Madan village of Khorasan.
Turquoise is treated to enhance both its colour and durability (i.e., increased hardness and decreased porosity). As is so often the case with any precious stones, full disclosure about treatment is frequently not given. It is therefore left to gemologists to detect these treatments in suspect stones using a variety of testing methods—some of which are necessarily destructive. For example, the use of a heated probe applied to an inconspicuous spot will reveal oil, wax, or plastic treatment with certainty.
Waxing and Oiling
Historically, light waxing and oiling were the first treatments used in ancient times, providing a wetting effect, thereby enhancing the colour and lustre. This treatment is more or less acceptable by tradition, especially because treated turquoise is usually of a higher grade to begin with. Oiled and waxed stones are prone to “sweating” under even gentle heat or if exposed to too much sun, and they may develop a white surface film or bloom over time. (With some skill, oil and wax treatments can be restored.)
Stabilization
Material treated with plastic or water glass is termed “bonded” or “stabilized” turquoise. This process consists of pressure impregnation of otherwise unsaleable chalky American material by epoxy and plastics (such as polystyrene) and water glass to produce a wetting effect and improve durability. Plastic and water glass treatments are far more permanent and stable than waxing and oiling, and can be applied to material too chemically or physically unstable for oil or wax to provide sufficient improvement. Conversely, stabilization and bonding are rejected by some as too radical an alteration.[14]
The epoxy binding technique was first developed in the 1950s and has been attributed to Colbaugh Processing of Arizona, a company that still operates today. The majority of American material is now treated in this manner although it is a costly process requiring many months to complete. Without such impregnation, most American mining operations would be unprofitable.
Dyeing
The use of Prussian blue and other dyes (often in conjunction with bonding treatments) to “enhance”—that is, make uniform or completely change—colour is regarded as fraudulent by some purists,[14] especially since some dyes may fade or rub off on the wearer. Dyes have also been used to darken the veins of turquoise.
Reconstitution
Perhaps the most radical of treatments is “reconstitution”, wherein fragments of fine turquoise material, too small to be used individually, are powdered and then bonded to form a solid mass. Much, if not all, of this “reconstituted” material is likely artificialwith no natural components, or may have foreign filler material added to it.
Irradiation
Not well known, but some turquoise is irradiated to become less “chalky”. This treatment is rarely disclosed. Like all irradiated gemstones, it should be tested by a Nuclear Regulatory Commission licensed laboratory before being sold in the USA.
Backing
Since finer turquoise is often found as thin seams, it may be glued to a base of stronger foreign material as a means of reinforcement. These stones are termed “Backed” and it is standard practice that all thinly cut turquoise in the Southwestern United States is backed.Native indigenous peoples of this region, because of their considerable use and wearing of turquoise, found that backing increased the durability of thinly cut slabs and cabs of turquoise. They observed that if the stone was not backed it would, for the most part, end up cracking. Early backing materials were the casings of old model T batteries and progressed to old phonograph records and most recently to the use of epoxy steel resins. Backing of turquoise is not known outside of the Native American and Southwestern United States jewelry trade. The value of turquoise of the highest quality is not discounted because it is backed and indeed the process is expected for most thinly cut American commercial gemstones.[citation needed]
Valuation and care

Slab of turquoise in matrix showing a large variety of different colouration
Hardness and richness of colour are two of the major factors in determining the value of turquoise; while colour is a matter of individual taste, generally speaking, the most desirable is a strong sky to “robin’s egg” blue (in reference to the eggs of the American Robin).[15]Whatever the colour, turquoise should not be excessively soft or chalky; even if treated, such lesser material (to which most turquoise belongs) is liable to fade or discolour over time and will not hold up to normal use in jewellery.
The mother rock or matrix in which turquoise is found can often be seen as splotches or a network of brown or black veins running through the stone in a netted pattern; this veining may add value to the stone if the result is complementary, but such a result is uncommon. Such material is sometimes described as “spiderweb matrix”; it is most valued in the Southwest United States and Far East, but is not highly appreciated in the Near East where unblemished and vein-free material is ideal (regardless of how complementary the veining may be). Uniformity of colour is desired, and in finished pieces the quality of workmanship is also a factor; this includes the quality of the polish and the symmetry of the stone. Calibrated stones—that is, stones adhering to standard jewellery setting measurements—may also be more sought after. Like coral and other opaque gems, turquoise is commonly sold at a price according to its physical size in millimetres rather than weight.
Turquoise is treated in many different ways, some more permanent and radical than others. Controversy exists as to whether some of these treatments should be acceptable, but one can be more or less forgiven universally: This is the light waxing or oiling applied to most gem turquoise to improve its colour and lustre; if the material is of high quality to begin with, very little of the wax or oil is absorbed and the turquoise therefore does not “rely” on this impermanent treatment for its beauty. All other factors being equal, untreated turquoise will always command a higher price. Bonded and “reconstituted” material is worth considerably less.
Being a phosphate mineral, turquoise is inherently fragile and sensitive to solvents; perfumeand other cosmetics will attack the finish and may alter the colour of turquoise gems, as will skin oils, as will most commercial jewelry cleaning fluids. Prolonged exposure to direct sunlight may also discolour or dehydrate turquoise. Care should therefore be taken when wearing such jewels: cosmetics, including sunscreen and hair spray, should be applied before putting on turquoise jewellery, and they should not be worn to a beach or other sun-bathed environment. After use, turquoise should be gently cleaned with a soft cloth to avoid a build up of residue, and should be stored in its own container to avoid scratching by harder gems. Turquoise can also be adversely affected if stored in an airtight container.
Topaz
opaz is a silicate mineral of aluminium and fluorine with the chemical formula Al2SiO4(F,OH)2. Topaz crystallizes in the orthorhombic system and its crystals are mostly prismatic terminated by pyramidal and other faces.
Color and varieties
Pure topaz is colorless and transparent but is usually tinted by impurities; typical topaz is wine, straw yellow, pale gray or reddish-orange. It can also be made white, pale green, blue, gold, pink (rare), reddish-yellow or opaque to transparent/translucent.
Orange topaz, also known as precious topaz, is the traditional November birthstone, the symbol of friendship, and the state gemstone for the US State of Utah. [5]
Imperial topaz is yellow, pink (rare, if natural) or pink-orange. Brazilian Imperial Topaz can often have a beautiful bright yellow to deep golden brown hue, sometimes even violet. Many brown or pale topazes are treated to make them bright yellow, gold, pink or violet colored. This variety is the most sought-after and highly valued among the topaz gems. Some imperial topaz stones can fade on exposure to sunlight for an extended period of time. [6][7]
Blue topaz is the Texas state gemstone.[8] Naturally occurring Blue Topaz is quite rare. Typically, colorless, gray or pale yellow and blue material is heat treated and irradiated in order to produce a more desired darker blue.[7]
Mystic topaz is colorless topaz which has been artificially coated giving it the desired rainbow effect.[9]
[edit] Localities and occurrence
Topaz is commonly associated with silicic igneous rocks of the granite and rhyolite type. It typically crystallizes in granitic pegmatites or in vapor cavities in rhyolite lava flows like those at Topaz Mountain in western Utah. It can be found with fluorite and cassiterite in varios areas including Ural and Ilmen mountains of Russia, in Afghanistan, Sri Lanka, Czech Republic,Germany, Norway, Pakistan, Italy, Sweden, Japan,Brazil, Mexico, Flinders Island and theUnited States.
Some clear topaz crystals from Brazilian pegmatites can reach boulder size and weigh hundreds of pounds. Crystals of this size may be seen in museum collections. The Topaz of Aurungzebe, observed by Jean Baptiste Tavernier measured 157.75 carats.[10]
Colorless and light-blue varieties of topaz are found in Precambrian granite in Mason County, Texas[11] within the Llano Uplift. There is no commercial mining of topaz in that area.[12]
Colorless topaz, Minas Gerais, Brazil
|
|
|
|
|
Etymology and historical and mythical usage
The name “topaz” is derived from the Greek Τοπάζιος (Τοpáziοs), the author of one of the first systematic treatises on minerals and gemstones dedicated two chapters on the topic in 1652.[13] In the Middle Ages, the name topaz was used to refer to any yellow gemstone, but now the name is only properly applied to the silicate described above.
Many modern English translations of the Bible, including the King James Version mentionTopaz in Exodus 28:17 in reference to a stone in the Hoshen: “And thou shalt set in it settings of stones, even four rows of stones: the first row shall be a sardius, a topaz, and a carbuncle(Garnet): this shall be the first row.”
However, since these translations as topaz all derive from the Septuagint translationtopazi[os], which as mentioned above referred to a yellow stone that was not topaz, but probably chrysolite, it should be borne in mind that topaz is not meant here.[14] The masoretic text (the Hebrew on which most modern Protestant Bible translations of the Old Testament are based) has pitdah as the gem the stone is made from; some scholars think it is related to an Assyrian word meaning flashed.[citation needed] More likely, pitdah is derived from Sanskrit words (pit = yellow, dah = burn), meaning “yellow burn”.
| Topaz |

A group of topaz crystals on matrix |
| General |
| Category |
Silicate mineral |
| Chemical formula |
Al2SiO4(F,OH)2 |
| Identification |
| Color |
Clear (if no impurities), blue, brown, orange, gray, yellow, green, pink and reddish pink. |
| Crystal system |
orthorhombic |
| Cleavage |
[001] Perfect |
| Fracture |
conchoidal |
| Mohs Scalehardness |
8 |
| Luster |
glassy |
| Streak |
white |
| Diaphaneity |
Transparent |
| Specific gravity |
3.49–3.57 |
| Optical properties |
Biaxial (+) |
| Refractive index |
nα = 1.606–1.629
nβ = 1.609–1.631
nγ = 1.616–1.638 |
| Birefringence |
δ = 0.010 |
| Pleochroism |
Weak in thick sections |
| Other characteristics |
Fluorescent, short UV=golden yellow, long UV=cream |
|
|
Opal
Opal is a mineraloid gel which is deposited at a relatively low temperature and may occur in the fissures of almost any kind of rock, being most commonly found with limonite, sandstone,rhyolite, marl and basalt. The word opal comes from the Latin opalus, byGreek opallios, and is from the same root as Sanskrit upálá[s] for “stone”, originally a millstone with upárá[s] for slab.[4]
The water content is usually between three and ten percent, but can be as high as twenty percent. Opal ranges from clear through white, gray, red, orange, yellow, green, blue, magenta, rose, pink, slate, olive, brown, and black. Of these hues, the reds against black are the most rare, whereas white and greens are the most common. These color variations are a function of growth size into the red and infrared wavelengths. Opal is Australia‘s national gemstone.
| Opal |

An opal bracelet. The stone size is 18 by 15 mm (0.7 by 0.6 inch). |
| General |
| Category |
Mineraloid |
| Chemical formula |
Hydrated silica. SiO2·nH2O |
| Identification |
| Color |
White, black, red, orange, most of the full spectrum, colorless,iridescent |
| Crystal habit |
Irregular veins, in masses, in nodules |
| Crystal system |
Amorphous[1] |
| Cleavage |
None[1] |
| Fracture |
Conchoidal to uneven[1] |
| Mohs Scalehardness |
5.5–6.5[1] |
| Luster |
Subvitreous to waxy[1] |
| Streak |
White |
| Diaphaneity |
opaque, translucent, transparent |
| Specific gravity |
2.15 (+.08, -.90)[1] |
| Polish luster |
Vitreous to resinous[1] |
| Optical properties |
Single refractive, often anomalous double refractive due to strain[1] |
| Refractive index |
1.450 (+.020, -.080) Mexican opal may read as low as 1.37, but typically reads 1.42–1.43[1] |
| Birefringence |
none[1] |
| Pleochroism |
None[1] |
| Ultravioletfluorescence |
black or white body color: inert to white to moderate light blue, green, or yellow in long and short wave. May also phosphoresce;common opal: inert to strong green or yellowish green in long and short wave, may phosphoresce;fire opal: inert to moderate greenish brown in long and short wave, may phosphoresce.[1] |
| Absorption spectra |
green stones: 660nm, 470nm cutoff[1] |
| Diagnostic features |
darkening upon heating |
| Solubility |
hot saltwater, bases,methanol,humic acid,hydrofluoric acid |
Precious opal
Precious opal shows a variable interplay of internal colors and even though it is a mineraloid, it does have an internal structure. At micro scales precious opal is composed of silica spheres some 150 to 300 nm in diameter in a hexagonal or cubic close-packedlattice. These ordered silica spheres produce the internal colors by causing the interference and diffraction of light passing through the microstructure of the opal.[5] It is the regularity of the sizes and the packing of these spheres that determines the quality of precious opal. Where the distance between the regularly packed planes of spheres is approximately half the wavelength of a component of visible light, the light of that wavelength may be subject to diffraction from thegrating created by the stacked planes. The spacing between the planes and the orientation of planes with respect to the incident light determines the colors observed. The process can be described by Bragg’s Law of diffraction.

Precious opal consists of spheres of silica of fairly regular size, packed into close-packed planes which are stacked together with characteristic dimensions of several hundred nm.
Visible light of diffracted wavelengths cannot pass through large thicknesses of the opal. This is the basis of the optical band gap in aphotonic crystal, of which opal is the best known natural example. In addition, microfractures may be filled with secondary silica and form thin lamellae inside the opal during solidification. The term opalescence is commonly and erroneously used to describe this unique and beautiful phenomenon, which is correctly termedplay of color. Contrarily, opalescence is correctly applied to the milky,turbid appearance of common or potch opal. Potch does not show a play of color.
The veins of opal displaying the play of color are often quite thin, and this has given rise to unusual methods of preparing the stone as a gem. An opal doublet is a thin layer of opal, backed by a swart mineral such as ironstone, basalt, or obsidian. The darker backing emphasizes the play of color, and results in a more attractive display than a lighter potch.
Combined with modern techniques of polishing, doublet opal produces similar effect of black or boulder opals at a mere fraction of the price. Doublet opal also has the added benefit of having genuine opal as the top visible and touchable layer, unlike triplet opals.
The triplet-cut opal backs the colored material with a dark backing, and then has a domed cap of clear quartz or plastic on top, which takes a high polish and acts as a protective layer for the relatively fragile opal. The top layer also acts as a magnifier, to emphasise the play of color of the opal beneath, which is often of lower quality. Triplet opals therefore have a more artificial appearance, and are not classed as precious opal.
Common opal
Besides the gemstone varieties that show a play of color, there are other kinds of common opal such as the milk opal, milky bluish to greenish (which can sometimes be of gemstone quality), resin opal which is honey-yellow with a resinous luster, wood opal which is caused by the replacement of the organic material in wood with opal[6], menilite which is brown or grey,hyalite is a colorless glass-clear opal sometimes called Muller’s Glass, geyserite, also calledsiliceous sinter, deposited around hot springs or geysers anddiatomite or diatomaceous earth, the accumulations of diatom shells or tests.
Other varieties of opal
Fire opals are transparent to translucent opals with warm body colors yellow, orange, orange-yellow or red and they do not show any play-of-color.. The most famous source of fire opals is the state of Queretaro in Mexico and these opals are commonly called Mexican fire opals.
Peruvian opal (also called blue opal) is a semi-opaque to opaque blue-green stone found in Peru which is often cut to include the matrix in the more opaque stones. It does not display pleochroism.

Boulder opal carving of a walrus, showing flashes of color from the exposed opal. The carving is 9 cm (3.5 inches) long.
Sources of opal

Polished opal from Yowah, Queensland, Australia
Australia produces around 97% of the world’s opal. 90% is called ‘light opal’ or white and crystal opal. White makes up 60% of the opal productions but cannot be found in all of the opal fields. Crystal opal or pure hydrated silica makes up 30% of the opal produced, 8% is black and only 2% is boulder opal.[citation needed]
The town of Coober Pedy in South Australia is a major source of opal. Andamooka in South Australia is also a major producer of matrix opal, crystal opal, and black opal. Another Australian town, Lightning Ridge in New South Wales, is the main source of black opal, opal containing a predominantly dark background (dark-gray to blue-black displaying the play of color). Boulder opal consists of concretions and fracture fillings in a dark siliceous ironstonematrix. It is found sporadically in western Queensland, from Kynuna in the north, to Yowah and Koroit in the south.[7]

Multi-colored rough opal specimen from Virgin Valley, Nevada, USA
The Virgin Valley opal fields of Humboldt County in northern Nevada produce a wide variety of precious black, crystal, white, fire, and lemon opal. The black fire opal is the official gemstone of Nevada. Most of the precious opal is partial wood replacement. Miocene age opalised teeth, bones, fish, and a snake head have been found. Some of the opal has high water content and may desiccate and crack when dried. The largest black opal in the Smithsonian Museumcomes from the Royal Peacock opal mine in the Virgin Valley.[citation needed]
Another source of white base opal in the United States is Spencer, Idaho. A high percentage of the opal found there occurs in thin layers. As a result, most of the production goes into the making of doublets and triplets.
Other significant deposits of precious opal around the world can be found in the Czech Republic, Slovakia, Hungary, Turkey, Indonesia, Brazil, Honduras, Guatemala, Nicaragua and Ethiopia.
In late 2008, NASA announced that it had discovered opal deposits on Mars.[8]
Synthetic opal
As well as occurring naturally, opals of all varieties have been synthesized experimentally and commercially. The discovery of the ordered sphere structure of precious opal led to its synthesis by Pierre Gilson in 1974.[5] The resulting material is distinguishable from natural opal by its regularity; under magnification, the patches of color are seen to be arranged in a “lizard skin” or “chicken wire” pattern. Synthetics are further distinguished from naturals by the former’s lack of fluorescence under UV light. Synthetics are also generally lower in density and are often highly porous.
Two notable producers of synthetic opal are the companies Kyocera and Inamori of Japan. Most so-called synthetics, however, are more correctly termed “imitation opal”, as they contain substances not found in natural opal (e.g., plastic stabilizers). The imitation opals seen in vintage jewelry are often “Slocum Stone” consisting of laminated glass with bits of foil interspersed.
Local atomic structure of opals
The lattice of spheres of opal that cause the interference with light are several hundred times larger than the fundamental structure of crystalline silica. As a mineraloid, there is no unit cellthat describes the structure of opal. Nevertheless, opals can be roughly divided into those that show no signs of crystalline order (amorphous opal) and those that show signs of the beginning of crystalline order, commonly termed cryptocrystalline or microcrystalline opal.[9]Dehydration experiments and infrared spectroscopy have shown that most of the H2O in the formula of SiO2·nH2O of opals is present in the familiar form of clusters of molecular water. Isolated water molecules, and silanols, structures such as Si-O-H, generally form a lesser proportion of the total and can reside near the surface or in defects inside the opal.
The structure of low-pressure polymorphs of anhydrous silica consist of frameworks of fully-corner bonded tetrahedra of SiO4. The higher temperature polymorphs of silica cristobalite andtridymite are frequently the first to crystallize from amorphous anhydrous silica, and the local structures of microcrystalline opals also appear to be closer to that of cristobalite andtridymite than to quartz. The structures of tridymite and cristobalite are closely related and can be described as hexagonal and cubic close-packed layers. It is therefore possible to have intermediate structures in which the layers are not regularly stacked.

The crystal structure of crystalline α-cristobalite. Locally, the structures of some opals, opal-C, are similar to this.
Microcrystalline opal
Opal-CT has been interpreted as consisting of clusters of stacking of cristobalite and tridymite over very short length scales. The spheres of opal in opal-CT are themselves made up of tiny microcrystalline blades of cristobalite and tridymite. Opal-CT has occasionally been further subdivided in the literature. Water content may be as high as 10 wt%. Lussatite is a synonym. Opal-C, also called Lussatine, is interpreted as consisting of localized order of α-cristobalite with a lot of stacking disorder. Typical water content is about 1.5wt%.
Non-crystalline opal
Two broad categories of non-crystalline opals, sometimes just referred to as “opal-A”, have been proposed. The first of these is opal-AG consisting of aggregated spheres of silica, with water filling the space in between. Precious opal and potch opal are generally varieties of this, the difference being in the regularity of the sizes of the spheres and their packing. The second “opal-A” is opal-AN or water-containing amorphous silica-glass. Hyalite is another name for this.
Non-crystalline silica in siliceous sediments is reported to gradually transform to opal-CT and then opal-C as a result of diagenesis, due to the increasing overburden pressure insedimentary rocks, as some of the stacking disorder is removed.[10]
Historical superstitions
In the Middle Ages, opal was considered a stone that could provide great luck because it was believed to possess all the virtues of each gemstone whose color was represented in the color spectrum of the opal.[11] Victorian superstitions were created by the established gem dealers to stop the rush to buy opals. They paid an author to attribute bad luck to the stone, though some believed this is avoided if opal is the owner’s birthstone or if the stone was a gift. Even as recently as under the last czar at the beginning of the 20th century, it was believed that when a Russian of any rank saw an opal among other goods offered for sale, he or she should not buy anything more since the opal was believed to embody the evil eye.[11] Opal is considered the birthstone for people born in October.
Peridot
Peridot (pronounced /ˈpɛrɪdɒt/ or /ˈpɛrɪdoʊ/) is gem-quality forsteritic olivine. The chemical composition of peridot is (Mg, Fe)2SiO4, with Mg in greater quantities than Fe.
The origin of the name “peridot” is uncertain. The Oxford English Dictionary suggests an alteration of Anglo-Norman pedoretés(classical Latin paederot-), a kind of opal, rather than the Arabic word faridat, meaning “gem”.
Olivine in general is a very abundant mineral, but gem quality peridot is rather rare.
Peridot is one of the few gemstones that occur in only one color: basically an olive green. The intensity and tint of the green however depends on how much iron is contained in the crystal structure, so the color of individual peridot gems can vary from yellow-green through olive green to brownish green. The most valuable is considered a dark-olive green color.
Peridot crystals have been collected from some Pallasite meteorites. A famous Pallasite was offered for auction in April 2008 with a requested price of close to $ 3 million at Bonhams, but remained unsold.[1]Peridot is the only gemstone found in meteorites.
Peridot olivine is the birthstone for August. It is sometimes mistaken for emeralds and other green gems. In fact notable gemologist George Frederick Kunz [2] discussed the confusion between emeralds and peridots in many church treasures, notably the “Three Magi” treasure in the Dom of Cologne, Germany.
Occurrence
Olivine, of which peridot is a type, is a common mineral in mafic and ultramafic rocks, and it is often found in lavas and in peridotitexenoliths of the mantle, which lavas carry to the surface; but gem quality peridot only occurs in a fraction of these settings.
Peridot olivine is mined in North Carolina, Arizona, Hawaii, Nevada, and New Mexico, in the US; and inAustralia, Brazil, China,Kenya, Mexico, Myanmar (Burma), Norway, Pakistan, South Africa, Sri Lanka, andTanzania. High quality peridot olivine is mined in the eastern lava fields of Saudi Arabia. However the best quality gems are considered to come from Pakistan and most other Peridot is now mined by Native Americans in the San Carlos Reservation in Arizona.[3]
In much antique jewelry, peridot could have come from Egypt: in the late 18th/early 19th century, peridot was taken from Egyptian ecclestial and other ornaments and reused in jewelry. Furthermore a location in Egypt was (re-) discovered but its location remains unknown.[4]
The largest cut peridot olivine is a 310 carat (62 g) specimen in the Smithsonian Museum in Washington, D.C..
Peridot olivine with minorpyroxene, on vesicularbasalt. (field of view = 60mm)
|
Peridot from the San Carlos Apache reservation in Arizona.
|
|
Peridot with milky inclusions
|
|
Pearl
A pearl is a hard, roundish object produced within the soft tissue (specifically the mantle) of a living shelled mollusk. Just like the shell of mollusks, a pearl is made up of calcium carbonatein minute crystalline form, which has been deposited in concentric layers. The ideal pearl is perfectly round and smooth, but many other shapes of pearls (baroque pearls) occur. The finest quality natural pearls have been highly valued as gemstones and objects of beauty for many centuries, and because of this, the word pearl became a metaphor for something very rare, very fine, very admirable and very valuable.
Valuable pearls occur in the wild, but they are very rare. Cultured or farmed pearls make up the majority of those that are currently sold. Pearls from the sea are valued more highly than freshwater pearls. Imitation or fake pearls are also widely sold in inexpensive jewelry, but the quality of the iridescence is usually very poor, and generally speaking, fake pearls are usually quite easy to distinguish from the real thing. Pearls have been harvested, or more recently cultivated, primarily for use in jewelry, but in the past they were also stitched onto lavish clothing, as worn, for example, by royalty. Pearls have also been crushed and used in cosmetics, medicines, or in paint formulations.
Pearls that are considered to be of gemstone quality are almost always nacreous andiridescent like the interior of the shell that produces them. However almost all species of shelled mollusks are capable of producing calcareous concretions. Although these may also be legitimately referred to as “pearls” under Federal Trade Commission rules[1], and are formed in the same way, most of them have no value, except as curios.
In several European languages, the word “pearl” is synonymous with “bead”, which can lead to confusion during translation.
Definition of a pearl

A black pearl and a shell of the black-lipped pearl oyster

Saltwater pearl oyster farm, Seram, Indonesia
Almost any shelled mollusk can, by natural processes, produce some kind of “pearl” when an irritating microscopic object becomes trapped within the mollusk’s mantle folds, but the great majority of these “pearls” are not valued as gemstones. Nacreous pearls, the best-known and most commercially-significant pearls, are primarily produced by two groups of molluscanbivalves or clams. A nacreous pearl is made from layers of nacre, by the same living process as is used in the secretion of the mother of pearl which lines the shell.
A “natural pearl” is one that forms without any human intervention at all, in the wild, and is very rare. Many hundreds of pearl oysters or pearl mussels have to be gathered and opened, and thus killed, in order to find even one wild pearl, and for many centuries that was the only way pearls were obtained. This was the main reason why pearls fetched such extraordinary prices in the past. Acultured pearl, on the other hand, is one that has been formed with human intervention on a pearl farm. The vast majority of pearls on the market today are cultured pearls.[citation needed]
One family of nacreous pearl bivalves, the pearl oysters, lives in the sea while the other, very different group of bivalves live in freshwater; these are the river mussels such as the freshwater pearl mussel. Saltwater pearls can grow in several species of marinepearl oysters in thefamily Pteriidae. Freshwater pearls grow within certain (but by no means all) species of freshwater mussels in the order Unionida, the families Unionidae and Margaritiferidae.
Physical properties

Akoya pearl grafting shed in Xuwen, China.
The unique luster of pearls depends upon the reflection, refraction, and diffraction of light from the translucent layers. The thinner and more numerous the layers in the pearl, the finer the luster. The iridescence that pearls display is caused by the overlapping of successive layers, which breaks up light falling on the surface.
In addition, pearls (especially cultured freshwater pearls) can be dyed yellow, green, blue, brown, pink, purple, or black.
Freshwater and saltwater pearls
Freshwater and saltwater pearls may sometimes look quite similar, but they come from different sources.
Natural freshwater pearls form in various species of freshwater mussels, family Unionidae, which live in lakes, rivers, ponds and other bodies of fresh water. These freshwater pearl mussels occur not only in hotter climates, but also in colder more temperate areas such asScotland: see the freshwater pearl mussel. However, most freshwater cultured pearls sold today come from China.
Saltwater pearls grow within pearl oysters, family Pteriidae, which live in oceans. Saltwaterpearl oysters are usually cultivated in protected lagoons or volcanic atolls.
Creation of a pearl

Diagram comparing a cross-section of a cultured pearl, upper, with a natural pearl, lower
The difference between natural and cultured pearls focuses on whether the pearl was created spontaneously by nature — without human intervention — or with human aid. Pearls are formed inside the shell of certain mollusks: as a defense mechanism to a potentially threatening irritant such as a parasite inside its shell, the mollusk creates a pearl to seal off the irritation.
The mantle of the mollusk deposits layers of calcium carbonate (CaCO3) in the form of themineral aragonite or a mixture of aragonite and calcite (both crystalline forms of calcium carbonate) held together by an organic horn-like compound called conchiolin. The combination of aragonite and conchiolin is called nacre, which makes up mother-of-pearl. The commonly held belief that a grain of sand acts as the irritant is in fact rarely the case. Typical stimuli include organic material, parasites, or even damage that displaces mantle tissue to another part of the animal’s body. These small particles or organisms enter the animal when the shell valves are open for feeding or respiration. In cultured pearls, the irritant is typically a cut piece of the mantle epithelium, together with processed shell beads, the combination of which the animal accepts into its body. [2][3][4]
Natural pearls
Natural pearls are nearly 100% calcium carbonate and conchiolin. It is thought that natural pearls form under a set of accidental conditions when a microscopic intruder or parasite enters a bivalve mollusk, and settles inside the shell. The mollusk, being irritated by the intruder, secretes the calcium carbonate and conchiolin to cover the irritant. This secretion process is repeated many times, thus producing a pearl. Natural pearls come in many shapes, with perfectly round ones being comparatively rare.
Cultured pearls
Cultured pearls (nucleated and non-nucleated or tissue nucleated cultured pearls) and imitation pearls can be distinguished from natural pearls by X-ray examination. Nucleated cultured pearls are often ‘pre-formed’ as they tend to follow the shape of the implanted shell bead nucleus. Once the pre-formed beads are inserted into the oyster, it secretes a few layers of nacre around the outside surface of the implant before it is removed after six months or more.
When a nucleated cultured pearl is X-rayed, it reveals a different structure to that of a natural pearl. A cultured pearl shows a solid center with no concentric growth rings, whereas a natural pearl shows a series of concentric growth rings.
Gemological identification
A well equipped gem testing laboratory (e.g. SSEF, Guebelin, GIA, AGTA, HIRCO-INDIA) is able to distinguish natural pearls from cultured pearls by using a gemological x-ray in order to examine the center of a pearl. With an x-ray it is possible to see the growth rings of the pearl, where the layers of calcium carbonate are separated by thin layers of conchiolin. The differentiation of natural pearls from tissue-nucleated cultured pearls can be very difficult without the use of this x-ray technique.
Natural and cultured pearls can be distinguished from imitation pearls using a microscope. Another method of testing for imitations is to rub the pearl against the surface of a front tooth. Imitation pearls are completely smooth, but natural and cultured pearls are composed of nacre platelets, which feel slightly gritty.
Value of a natural pearl
Quality natural pearls are very rare jewels. The actual value of a natural pearl is determined in the same way as it would be for other “precious” gems. The valuation factors include size, shape, quality of surface, orient and luster.
Single natural pearls are often sold as a collector’s item, or set as centerpieces in unique jewelry. Very few matched strands of natural pearls exist, and those that do often sell for hundreds of thousands of dollars. (In 1917, jeweler Pierre Cartier purchased the Fifth Avenue mansion that is now the New York Cartier store for US$100 cash and a double strand of matched natural pearls valued at the time at US$1 million.)
Keshi pearls, although they often occur by chance, are not considered natural pearls. They are a byproduct of the culturing process, and hence do not happen without human intervention. These pearls are quite small: typically a few millimeters in size. Keshi pearls are produced by many different types of marine mollusks and freshwater mussels in China.[5] Today many “keshi” pearls are actually intentional, with post-harvest shells returned to the water to regenerate a pearl in the existing pearl sac.
Origin of a natural pearl
Previously, natural pearls were found in many parts of the world. Present day natural pearling is confined mostly to seas off Bahrain. Australia also has one of the world’s last remaining fleets of pearl diving ships. Australian pearl divers dive for south sea pearl oysters to be used in the cultured south sea pearl industry. The catch of pearl oysters is similar to the numbers of oysters taken during the natural pearl days. Hence significant numbers of natural pearls are still found in the Australian Indian Ocean waters from wild oysters. X-Ray examination is required to positively verify natural pearls found today.
Different types of cultured pearls, including black pearls

A blister pearl, a half-sphere, formed flush against the shell of the pearl oyster
Black pearls, frequently referred to as Black Tahitian Pearls, are highly valued because of their rarity; the culturing process for them dictates a smaller volume output and can never be mass produced.[citation needed] This is due to bad health and/or non-survival of the process, rejection of the nucleus and their sensitivity to changing climatic and ocean conditions. Before the days of cultured pearls, black pearls were rare and highly valued for the simple reason that white pearl oysters rarely produced naturally black pearls, and black pearl oysters rarely produced any natural pearls at all.
Since the development of pearl culture technology, the black pearl oyster found in Tahiti and many other Pacific Island areas has been extensively used for producing cultured pearls. The rarity of the black cultured pearl is now a “comparative” issue. The black cultured pearl is rare when compared to Chinese freshwater cultured pearls, and Japanese and Chinese akoya cultured pearls, and is more valuable than these pearls. However, it is more abundant than the South Sea pearl, which is more valuable than the black cultured pearl. This is simply because the black pearl oyster Pinctada margaritifera is far more abundant than the elusive, rare, and larger south sea pearl oyster Pinctada maxima, which cannot be found in lagoons, but which must be dived for in a rare number of deep ocean habitats or grown in hatcheries.
Black cultured pearls from the black pearl oyster — Pinctada margaritifera — are not South Sea pearls, although they are often mistakenly described as black South Sea pearls. In the absence of an official definition for the pearl from the black oyster, these pearls are usually referred to as “black Tahitian pearls”.
The correct definition of a South Sea pearl — as described by CIBJO and GIA — is a pearl produced by the Pinctada maxima pearl oyster. South Sea pearls are the color of their hostPinctada maxima oyster — and can be white, silver, pink, gold, cream, and any combination of these basic colors, including overtones of the various colors of the rainbow displayed in the pearl nacre of the oyster shell itself.
Pearls and calcareous concretions from other species
Biologically speaking, under the right set of circumstances, almost any shelled mollusk can produce some kind of pearl, however, most of these molluscan pearls have no luster oriridescence. The great majority of mollusk species produce pearls which are not attractive to look at, and are sometimes not even very durable, such that they usually have no value at all, except perhaps to a scientist, a collector, or as a curiosity. These objects used to be referred to as “calcareous concretions” by some gemologists, even though a malacologist would still consider them to be pearls. Valueless pearls of this type are sometimes found in ediblemussels, edible oysters, escargot snails, and so on. Kenneth Scarrat, director of GIA Bangkok, has recently argued for changes to current nomenclature. He argues conch “pearls” should be referred to (and various other types of mollusc pearls) as simply pearls, not “calcareous concretions”.[6]. Under Federal Trade Commission rules, various mollusc pearls may be referred to as ‘pearls’ without qualification. [7]
A few species produce pearls that can be of interest as gemstones. These species include the bailer shell Melo (genus), the giant clam Tridacna, various scallop species, Pen shellsPinna (genus), and abalones. Another example is the conch pearl (sometimes referred to simply as the ‘pink pearl’), which is found very rarely growing between the mantle and the shell of the queen conch or pink conch, Strombus gigas, a large sea snail or marine gastropod from the Caribbean Sea. These pearls, which are often pink in color, are a by-product of the conch fishing industry, and the best of them display a shimmering optical effect related tochatoyance known as ‘flame structure’.
Somewhat similar gastropod pearls, this time more orange in hue, are (again very rarely) found in the horse conch Pleuroploca gigantea.

Largest known pearl from a giant clam
The largest pearl known was found in the Philippines in 1934. It is a naturally-occurring, non-nacreous, calcareous concretion (pearl) from a giant clam. Because it did not grow in a pearl oyster it is not pearly, instead it has a porcellaneous surface. In other words, it is glossy like a china plate. Other pearls from giant clams are known to exist, but this is a particularly large one.. The pearl weighs 14 lb (6.4 kg) and was supposedly first discovered by an anonymousFilipino Muslim diver off the island of Palawan in 1934. According to the legend as it is currently told, a Palawan chieftain gave the pearl to Wilbur Dowell Cobb in 1936 as a gift for having saved the life of his son. The pearl had been named the “Pearl of Allah” by the Muslim tribal chief, because it resembled a turbaned head. Another even more elaborate legend says that this object is actually the Pearl of Lao-Tzu, a cultured pearl created with a carved amulet and then supposedly progressively grafted into several giant clams, before supposedly being lost due to a shipwreck in 1745. [8] This legend has been discredited, however because this pearl is indeed the product of a giant clam, Tridacna gigas, which cannot be grafted. The pearl is also a whole pearl, not a mabe pearl, and whole pearl culturing technology is only 100 years old. [9]
The history of pearl hunting and pearl farming
Pearl hunting
For thousands of years, most seawater pearls were retrieved by divers working in the Indian Ocean, in areas like the Persian Gulf, the Red Sea, and in the Gulf of Mannar.[citation needed]
Starting in the Han Dynasty (206 BC – 220 AD), the Chinese hunted extensively for seawater pearls in the South China Sea.[citation needed]
When Spanish conquistadors arrived in the Western Hemisphere, they discovered that around the islands of Cubagua and Margarita, some 200 km north of the Venezuelan coast, was an extensive pearl bed. One discovered and named pearl, La Peregrina, was offered to the Spanish queen.[citation needed] According to Garcilasso de la Vega, who says that he saw La Peregrina at Seville in 1507, (Garcilasso, “Historie des Incas, Rois du Perou,” Amsterdam, 1704, Vol. II, P. 352.) this was found at Panama in 1560 by a negro who was rewarded with his liberty, and his owner with the office of alcalde of Panama.
Margarita pearls are extremely difficult to find today and are known for their unique yellowish color. The most famous Margarita necklace that any one can see today is the one that then Venezuelan President Romulo Betancourt gave to Jacqueline Kennedywhen she and her husband, President John F. Kennedy paid an official visit to Venezuela.[citation needed]
Before the beginning of the 20th Century, pearl hunting was the most common way of harvesting pearls. Divers manually pulled oysters from ocean floors and river bottoms and checked them individually for pearls. Not all mussels and oysters produce pearls. In a haul of three tons, only three or four oysters will produce perfect pearls[citation needed].
The development of pearl farming
Today, almost all pearls used for jewelry are cultured by planting a core or nucleus into pearl oysters. The pearls are usually harvested after one year for akoya, 2–4 years for Tahitian and South Sea, and 2–7 years for freshwater. This perliculture process was first developed byWilliam Saville-Kent who passed the information along to Tatsuhei Mise and Tokichi Nishikawa from Japan.
The nucleus is generally a polished bead made from freshwater mussel shell. Along with a small piece of mantle tissue from another mollusk to serve as a catalyst for the pearl sac, it is surgically implanted into the gonad (reproductive organ) of a saltwater mollusk. In freshwater perliculture, only the piece of tissue is used in most cases, and is inserted into the fleshy mantle of the host mussel. South Sea and Tahitian pearl oysters, also known as Pinctada maxima and Pinctada margaritifera, which survive the subsequent surgery to remove the finished pearl, are often implanted with a new, larger nucleus as part of the same procedure and then returned to the water for another 2–3 years of growth.
 Despite the common misperception, Mikimoto did not discover the process of pearl culture. The accepted process of pearl culture was developed by William Saville-Kent in Australia and brought to Japan by Tokichi Nishikawa and Tatsuhei Mise. Nishikawa was granted the patent in 1916, and married the daughter of Mikimoto. Mikimoto was able to use Nishikawa’s technology. After the patent was granted in 1916, the technology was immediately commercially applied to akoya pearl oysters in Japan in 1916. Mise’s brother was the first to produce a commercial crop of pearls in the akoya oyster. Mitsubishi’s Baron Iwasaki immediately applied the technology to the south sea pearl oyster in 1917 in the Philippines, and later in Buton, and Palau. Mitsubishi was the first to produce a cultured south sea pearl — although it was not until 1928 that the first small commercial crop of pearls was successfully produced.
The original Japanese cultured pearls, known as akoya pearls, are produced by a species of small pearl oyster, Pinctada fucata martensii, which is no bigger than 6 to 8 cm in size, hence akoya pearls larger than 10 mm in diameter are extremely rare and highly prized. Today, a hybrid mollusk is used in both Japan and China in the production of akoya pearls. It is a cross between the original Japanese species, and the Chinese species Pinctada chemnitzii.[10]
Recent pearl production
China has recently overtaken Japan in akoya pearl production. Japan has all but ceased its production of akoya pearls smaller than 8 mm. Japan maintains its status as a pearl processing center, however, and imports the majority of Chinese akoya pearl production. These pearls are then processed (often simply matched and sorted), relabeled as product of Japan, and exported.[11]
In the past couple of decades, cultured pearls have been produced using larger oysters in the south Pacific and Indian Ocean. The largest pearl oyster is the Pinctada maxima, which is roughly the size of a dinner plate. South Sea pearls are characterized by their large size and warm luster. Sizes up to 14 mm in diameter are not uncommon. South Sea pearls are primarily produced in Australia,Indonesia and the Philippines.
Mitsubishi commenced pearl culture with the south sea pearl oyster in 1916, as soon as the technology patent was commercialized. By 1931 this project was showing signs of success, but was upset by the death of Tatsuhei Mise. Although the project was recommenced after Tatsuhei’s death, the project was discontinued at the beginning of WWII before significant productions of pearls were achieved.
After WWII, new south sea pearl projects were commenced in the early 1950s in Burma andKuri Bay and Port Essington in Australia. Japanese companies were involved in all projects using technicians from the original Mitsubishi south sea pre-war projects.
Japanese freshwater pearl farming
In 1914, pearl farmers began growing cultured freshwater pearls using the pearl mussels native to Lake Biwa. This lake, the largest and most ancient in Japan, lies near the city of Kyoto. The extensive and successful use of the Biwa Pearl Mussel is reflected in the name Biwa pearls, a phrase which was at one time nearly synonymous with freshwater pearls in general.. Since the time of peak production in 1971, when Biwa pearl farmers produced six tons of cultured pearls, pollution has caused the virtual extinction of the industry. Japanese pearl farmers recently cultured a hybrid pearl mussel — a cross between Biwa Pearl Mussels and a closely related species from China, Hyriopsis cumingi, in Lake Kasumigaura. This industry has also nearly ceased production, due to pollution.
Japanese pearl producers also invested in producing cultured pearls with freshwater musselsin the region of Shanghai, China. China has since become the world’s largest producer of freshwater pearls, producing more than 1,500 metric tons per year.
Led by pearl pioneer John Latendresse and his wife Chessy, the United States began farmingcultured freshwater pearls in the mid 1960′s. National Geographic Magazine introduced the American cultured pearl as a commercial product in their August 1985 issue. The Tennessee pearl farm has emerged as a tourist destination in recent years, but commercial production of freshwater pearls has ceased.
Pearls in jewelry
The value of the pearls in jewelry is determined by a combination of the luster, color, size, lack of surface flaw and symmetry that are appropriate for the type of pearl under consideration. Among those attributes, luster is the most important differentiator of pearl quality according to jewelers.
All factors being equal, however, the larger the pearl the more valuable it is. Large, perfectly round pearls are rare and highly valued. Teardrop-shaped pearls are often used in pendants.
Shapes
Pearls come in eight basic shapes: round, semi-round, button, drop, pear, oval, baroque, and circled. Perfectly round pearls are the rarest and most valuable shape. Semi-rounds are also used in necklaces or in pieces where the shape of the pearl can be disguised to look like it is a perfectly round pearl. Button pearls are like a slightly flattened round pearl and can also make a necklace, but are more often used in single pendants or earrings where the back half of the pearl is covered, making it look like a larger, round pearl.

Woman with a Pearl Necklace, by Jan Vermeer van Delft, 1665
Drop and pear shaped pearls are sometimes referred to as teardrop pearls and are most often seen in earrings, pendants, or as a center pearl in a necklace.. Baroque pearls have a different appeal to them than more standard shapes because they are often highly irregular and make unique and interesting shapes. They are also commonly seen in necklaces. Circled pearls are characterized by concentric ridges, or rings, around the body of the pearl.
In general, cultured pearls are less valuable than natural pearls, and imitation pearls are less valuable than cultured pearls. One way that jewelers can determine whether a pearl is cultured or natural is to have a gem lab perform an x-ray of the pearl. If the x-ray reveals a nucleus, the pearl is likely a bead-nucleated saltwater pearl. If no nucleus is present, but irregular and small dark inner spots indicating a cavity are visible, combined with concentric rings of organic substance, the pearl is likely a cultured freshwater.Cultured freshwater pearls can often be confused for natural pearls which present as homogeneous pictures which continuously darken toward the surface of the pearl. Natural pearls will often show larger cavities where organic matter has dried out and decomposed.
Some imitation pearls are simply made of mother-of-pearl, coral or conch shell, while others are made from glass and are coated with a solution containing fish scales called essence d’Orient. Although imitation pearls look the part, they do not have the same weight or smoothness as real pearls, and their luster will also dim greatly.
Lengths of pearl necklaces

Portrait of Caterina Sagredo Barbarigo by Rosalba Carriera, cir. 1740. The subject is wearing a single-strand pearl collar and pendant pearl earrings

Queen of Italy, Margherita of Savoy, owned one of the most famous collections of natural pearls. She is wearing a multi-strand choker and a rope of pearls, possibly with matching bracelet and earrings
There is a special vocabulary used to describe the length of pearl necklaces. While most other necklaces are simply referred to by their physical measurement, pearl necklaces are named by how low they hang when worn around the neck. A collar, measuring 10 to 13 inches or 25 to 33 cm in length, sits directly against the throat and does not hang down the neck at all; collars are often made up of multiple strands of pearls. Pearl chokers, measuring 14 to 16 inches or 35 to 41 cm in length, nestle just at the base of the neck. A strand called a princess length, measuring 17 to 19 inches or 43 to 48 cm in length, comes down to or just below the collarbone. A matinee length, measuring 20 to 24 inches or 50 to 60 cm in length, falls just above the breasts. An opera length, measuring 28 to 35 inches or 70 to 90 cm in length, will be long enough to reach the breastbone or sternum of the wearer; and longer still, a pearl rope, measuring more than 45 inches or 115 cm in length, is any length that falls down farther than an opera.
Necklaces can also be classified as uniform, or graduated. In a uniform strand of pearls, all pearls are classified as the same size, but actually fall in a range. A uniform strand of akoya pearls, for example, will measure within 0.5 mm. So a strand will never be 7 mm, but will be 6.5-7 mm. Freshwater pearls, Tahitian pearls, and South Sea pearls all measure to a full millimeter when considered uniform.
A graduated strand of pearls most often has at least 3 mm of differentiation from the ends to the center of the necklace. Popularized in the United States during the 1950s by the GIsbringing strands of cultured akoya pearls home from Japan, a 3.5 momme, 3 mm to 7 mm graduated strand was much more affordable than a uniform strand because most of the pearls were small.
Colors of pearl jewelry
Earrings and necklaces can also be classified on the grade of the color of the pearl. While white, and more recently black, saltwater pearls are by far the most popular, other color tints can be found on pearls from the oceans. Pink, blue, champagne, green and even purple saltwater pearls can be encountered, but to collect enough pearls to form a complete string of the same size and same shade can take years.
Religious references
Hebrew scriptures
According to Rebbenu Bachya, the word Yahalom in the verse Exodus 28:18 means “pearl” and was the stone on the Hoshenrepresenting the tribe of Zebulun. This is generally disputed among scholars, particularly since the word in question in most manuscripts is actuallyYasepheh – the word from which jasper derives; scholars think that refers to green jasper (the rarest and most prized form in early times) rather than red jasper (the most common form).Yahalom is usually translated by the Septuagint as an “onyx“, but sometimes as “beryl” or as “jasper”; onyx only started being mined after the Septuagint was written, so the Septuagint’s term “onyx” probably does not mean onyx — onyx is originally an Assyrian word meaning ring, and so could refer to anything used for making rings. Yahalom is similar to a Hebrew word meaning hit hard, so some people think that it means diamond. The variation in possibilities of meaning for this sixth stone in the Hoshen is reflected in different translations of the Bible — the King James Version translates the sixth stone as diamond, the New International Version translates it as emerald, and the Vulgate translates it as jaspis — meaning jasper. There is a wide range of views among traditional sources about which tribe the stone refers to.
New Testament scriptures

Religious pendant showing Christ blessing, framed with rubies and pearls, from theByzantine empire, 12th or 13th century
In a Christian New Testament parable, Jesus compared the Kingdom of Heaven to a “pearl of great price” in Matthew 13: 45-46. “Again, the kingdom of heaven is like unto a merchant man, seeking goodly pearls: Who, when he had found one pearl of great price, went and sold all that he had, and bought it.”
The language of symbolism was in common use around the time of Jesus Christ; most people were familiar with the symbolic meanings. The circle is a symbol of God because it has no beginning and no end. The circle or pearl was considered to represent Love, Knowledge (the combination of equal amounts of Love and Knowledge is a symbol of Wisdom, the 2 circles intertwined (owl eyes) is symbolic of Wisdom. Some other pearls are Truth, and Faith.
The twelve gates of the New Jerusalem are reportedly each made of a single pearl inRevelation 21:21, that is, the Pearly Gates.. “And the twelve gates were twelve pearls; every gate was of one pearl: and the streets of the city were pure gold, as if transparent glass.”
Holy things are compared to pearls in Matthew 7:6. “Give not that which is holy unto the dogs, neither cast ye your pearls before swine, lest they trample them under their feet, and turn again and rend you.”
Pearls are also found in numerous references showing the wickedness and pride of a people, as in Revelation 18:16. “And saying, Alas, alas, that great city, that was clothed in fine linen, and purple, and scarlet, and decked with gold, and precious stones, and pearls!”
Islamic scriptures
The Qur’an often mentions that dwellers of paradise will be adorned with pearls:
22:23 God will admit those who believe and work righteous deeds, to Gardens beneath which rivers flow: they shall be adorned therein with bracelets of gold and pearls; and their garments there will be of silk.
35:33 Gardens of Eternity will they enter: therein will they be adorned with bracelets of gold and pearls; and their garments there will be of silk.
The handsome young boys in paradise are similarly depicted:
52:24 Round about them will serve, [devoted] to them, youths [handsome] as pearls well-guarded.
Hindu scriptures
The Vedic tradition describes the sacred Nine Pearls which were first documented in theGaruda Purana, one of the books of the Hindu holy text Atharvaveda. Ayurveda contains references to pearl powder as a stimulant of digestion and to treat mental ailments. According to Marco Polo, the kings of Malabar (now known as the Coromandel Coast) wore a necklace of 108 rubies and 108 precious pearls which was given from one generation of kings to the next.. The reason was that every king had to say 108 prayers every morning and every evening.[12] At least until the beginning of the 20th century it was a Hindu custom to present a completely new, undrilled pearl and pierce it during the ceremony.[12]
The Pearl or Mukta in Sanskrit is also associated with many Hindu deities. The most famous being the Koustubha which Lord Vishnu wears on his chest. Apart from religious connotations, stories and folklore abound of pearls occurring in snakes, the Naaga Mani, and elephants, the Gaja Mukta.
Other scriptures
The metaphor of a pearl appears in the longer Hymn of the Pearl, a poem respected for its high literary quality, and use of layered theological metaphor, found within one of the texts ofGnosticism.
The Pearl of Great Price is a book of scripture in The Church of Jesus Christ of Latter-day Saints. |
Emerald
Emeralds are a variety of the mineral beryl (Be3Al2(SiO3)6,) colored green by trace amounts of chromium and sometimesvanadium.[1] Beryl has a hardness of 7.5 – 8 on the 10 pointMohs scale of mineral hardness.[1] Most emeralds are highly included, so their toughness (resistance to breakage) is classified as generally poor. The word “emerald” comes from Latinsmaragdus, via Greeksmaragdos, its original source being a Semitic word izmargad or theSanskrit word, marakata, meaning “emerald” or “green”.[2]
Emeralds are a variety of the mineral beryl (Be3Al2(SiO3)6,) colored green by trace amounts of chromium and sometimesvanadium.[1] Beryl has a hardness of 7.5 – 8 on the 10 pointMohs scale of mineral hardness.[1] Most emeralds are highly included, so their toughness (resistance to breakage) is classified as generally poor. The word “emerald” comes from Latinsmaragdus, via Greeksmaragdos, its original source being a Semitic word izmargad or theSanskrit word, marakata, meaning “emerald” or “green”.[2]
Properties determining value
Emeralds, like all colored gemstones, are graded using four basic parameters, the four Cs of Connoisseurship; Color, Cut, Clarityand Crystal. The last C, crystal is simply used as a synonym that begins with C for transparency or what gemologists calldiaphaneity. Prior to the 20th Century jewelers used the term water as in “a gem of the finest water”[3] to express the combination of two qualities, color and crystal. Normally, in the grading of colored gemstones, color is by far the most important criterion. However, in the grading of emerald, crystal is considered a close second. Both are necessary conditions. A fine emerald must possess not only a pure verdant green hue as described below, but also a high degree of transparency to be considered a top gem.[4]
Color
Scientifically speaking, color is divided into three components: hue, saturation and tone. Yellow and blue, the hues found adjacent to green on the spectral color wheel, are the normal secondary hues found in emerald. Emeralds occur in hues ranging from yellowish green to bluish green. The primary hue must, of course, be green. Only gems that are medium to dark in tone are considered emerald. Light toned gems are known by the species name, green beryl. In addition, the hue must be bright (vivid). Gray is the normal saturation modifier or mask found in emerald. A grayish green hue is a dull green hue.
Clarity
Emerald tends to have numerous inclusions and surface breaking fissures. Unlike diamond, where the loupe standard, i.e. 10X magnification, is used to grade clarity, emerald is graded by eye. Thus, if an emerald has no visible inclusions to the eye (assuming normal visual acuity) it is considered flawless. Stones that lack surface breaking fissures are extremely rare and therefore almost all emeralds are treated, “oiled”, to enhance the apparent clarity. Eye-clean stones of a vivid primary green hue (as described above) with no more than 15% of any secondary hue or combination (either blue or yellow) of a medium-dark tone command the highest prices.[4] This relative crystal non-uniformity makes emeralds more likely than other gemstones to be cut into cabochons, rather than faceted shapes.
Treatments
Most emeralds are oiled as part of the post lapidary process, in order to improve their clarity.Cedar oil, having a similar refractive index, is often used in this generally accepted practice. Other liquids, including synthetic oils and polymers with refractive indexes close to that of emerald such as Opticon are also used. The U.S. Federal Trade Commission requires the disclosure of this treatment when a treated emerald is sold.[5] The use of oil is traditional and largely accepted by the gem trade. Other treatments, for example the use of green-tinted oil, are not acceptable in the trade. The laboratory community has recently standardized the language for grading the clarity of emeralds. Gems are graded on a four step scale; none,minor, moderate and highly enhanced. Note that these categories reflect levels of enhancement not clarity. A gem graded none on the enhancement scale may still exhibit visible inclusions. Laboratories tend to apply these criteria differently. Some gem labs consider the mere presence of oil or polymers to constitute enhancement. Others may ignore traces of oil if the presence of the material does not materially improve the look of the gemstone.
Given that the vast majority of all emeralds are treated as described above, and the fact that two stones that appear to be similar in quality may actually be quite far apart in treatment level, a consumer considering a purchase of an expensive emerald is well advised to insist upon a treatment report from a reputable gemological laboratory. All other factors being equal, a high quality emerald with an enhancement level graded moderate should cost 40-50% less than an identical stone graded none.
Emerald localities
Emeralds in antiquity were mined by the Egyptians and in Austria, as well as Swat in northernPakistan.[6][7]
A rare type of emerald known as a trapiche emerald is occasionally found in the mines ofColombia. A trapiche emerald exhibits a “star” pattern; it has raylike spokes of dark carbon impurities that give the emerald a six-pointed radial pattern. It is named for thetrapiche, a grinding wheel used to process sugarcane in the region. Colombian emeralds are generally the most prized due to their transparency and fire. Some of the most rare emeralds come from three main emerald mining areas in Colombia: Muzo, Coscuez, and Chivor. Fine emeralds are also found in other countries, such as Zambia, Brazil, Zimbabwe, Madagascar, Pakistan,India,Afghanistan and Russia. In the US, emeralds can be found in Hiddenite, North Carolina. In 1998, emeralds were discovered in theYukon.
Synthetic emerald

Emerald showing its hexagonal structure
Emerald is a rare and valuable gemstone and, as such, it has provided the incentive for developing synthetic emeralds. Both hydrothermal and flux-growth synthetics have been produced, and a method has been developed for producing an emerald overgrowth on colorless beryl. The first commercially successful emerald synthesis process was that of Carroll Chatham. Because Chatham’s emeralds do not have any water and contain traces of vanadate, molybdenum and vanadium, a lithium vanadate flux process is probably involved. The other large producer of flux emeralds was Pierre Gilson Sr., which has been on the market since 1964. Gilson’s emeralds are usually grown on natural colorless beryl seeds which become coated on both sides. Growth occurs at the rate of 1 mm per month, a typical seven-month growth run producing emerald crystals of 7 mm of thickness.[8] Gilson sold his production laboratory to a Japanese firm in the 1980s, but production has ceased since, so did Chatham’s, after the San Francisco earthquake in 1989.[citation needed]
Hydrothermal synthetic emeralds have been attributed to IG Farben, Nacken, Tairus, and others, but the first satisfactory commercial product was that of Johann Lechleitner ofInnsbruck, Austria, which appeared on the market in the 1960s. These stones were initially sold under the names “Emerita” and “Symeralds”, and they were grown as a thin layer of emerald on top of natural colorless beryl stones. Although not much is known about the original process, it is assumed that Leichleitner emeralds were grown in acid conditions.[citation needed] Later, from 1965 to 1970, the Linde Division of Union Carbideproduced completely synthetic emeralds by hydrothermal synthesis. According to their patents (US3,567,642 and US3,567,643), acidic conditions are essential to prevent the chromium (which is used as the colorant) from precipitating. Also, it is important that the silicon-containing nutrient be kept away from the other ingredients to prevent nucleation and confine growth to the seed crystals. Growth occurs by a diffusion-reaction process, assisted by convection. The largest producer of hydrothermal emeralds today is Tairus in Russia. They have succeeded to synthesize emeralds that have similar chemical composition as emeralds in alkaline deposits in Colombia, hence they are called “Colombian Created Emeralds” or “Tairus Created Emeralds.”[citation needed]
Luminescence in ultraviolet light is considered a supplementary test when making a natural vs. synthetic determination, as many, but not all, natural emeralds are inert to ultraviolet light. Many synthetics are also UV inert.[9]
Synthetic emeralds are often referred to as “created”, as their chemical and gemological composition is the same as their natural counterparts. The U.S. Federal Trade Commission(FTC) has very strict regulations as to what can and what cannot be called “synthetic” stone. The FTC says: “§ 23.23(c) It is unfair or deceptive to use the word “laboratory-grown,” “laboratory-created,” “[manufacturer name]-created,” or “synthetic” with the name of any natural stone to describe any industry product unless such industry product has essentially the same optical, physical, and chemical properties as the stone named.”[10]
Wispy veil-like inclusions are common in flux-grown synthetic emeralds.
Emerald in different cultures, and emerald lore
Emerald is regarded as the traditional birthstone for May, as well as the traditional gemstone for the astrological signs of Taurus,Cancer and sometimes Gemini. One of the more quaint anecdotes on emeralds was by the 16th-century historian Brantome, who referred to the many impressive emeralds the Spanish under Cortez had brought back to Europe from Latin America. On one of Cortez’s most notable emeralds he had the text engraved Inter Natos Mulierum non sur-rexit mayor (Among them borne of woman there hath not arisen a greater Man. XI, 11) which referred to John the Baptist. Brantome considered engraving such a beautiful and simple product of nature sacrilegious and considered this act the cause for Cortez’s loss of an extremely precious pearl (to which he dedicated a work A beautiful and incomparable pearl) and even for the death of King Charles IX who died soon after.[11]
In some cultures, the emerald is the traditional gift for the 55th wedding anniversary. It is also used as a 20th and 35th wedding anniversary stone.
The Authorized King James Version of the Bible, in Exodus 28:18 and 39:11, lists “emerald” as one of the precious stones in thebreastplate of the high priest of the Jews; but modern consensus is that this is probably a mistranslation. (See Hoshen.)
Ireland is often referred to, especially in America, as the “Emerald Isle”. |
Diamond
n mineralogy, diamond (from the ancient Greek adámas, meaning “proper” or “unalterable”) is an allotrope of carbon, where thecarbon atoms are arranged in a variation of the face centered cubic crystal structure called a diamond lattice. Diamond is the second most stable form of carbon, after graphite; however, the conversion rate from diamond to graphite is negligible at ambient conditions. Diamond is specifically renowned as a material with superlative physical qualities, most of which originate from the strong covalent bondingbetween its atoms. In particular, diamond has the highest hardness and thermal conductivityof any bulk material synthesized so far. Those properties determine the major industrial application of diamond in cutting and polishing tools.Diamond has remarkable optical characteristics. Because of its extremely rigid lattice, it can be contaminated by only few types of impurities, such as boron and nitrogen. Combined with the wide transparency (corresponding to the wide band gap of 5.5 eV), this results in clear, colorless appearance of most natural diamonds. Small amounts of defects or impurities (about one part per million) color diamond blue (boron), yellow (nitrogen), brown (lattice defects), green, purple, pink, orange or red. Diamond also has relatively high optical dispersion, that is ability to disperse light of different colors, which results in its characteristic luster. Excellent optical and mechanical properties, combined with efficient marketing, make diamond the most popular gemstone.
Most natural diamonds are formed at high-pressure high-temperature conditions existing at depths of 140 km to 190 km in the Earthmantle. Carbon-containing minerals provide the carbon source, and the growth occurs over periods from 1 billion to 3.3 billion years, which respectively corresponds to roughly 25% and 75% of the age of the Earth. Diamonds are brought close to the Earth surface through deep volcanic eruptions by a magma, which cools into igneous rocks known as kimberlites and lamproites. Diamonds can also be produced synthetically in a high-pressure high-temperature process which approximately simulates the conditions in the Earth mantle. An alternative, and completely different growth technique ischemical vapor deposition. Several non-diamond materials, which include cubic zirconia andsilicon carbide and are often called diamond simulants, resemble diamond in appearance and many properties. Special gemological techniques have been specially developed to distinguish natural and synthetic diamonds and diamond simulants.
| Diamond |

A scattering of round-brilliant cut diamonds shows off the many reflecting facets. |
| General |
| Category |
Native Minerals |
| Chemical formula |
C |
| Identification |
| Molar mass |
12.01 u |
| Color |
Typically yellow, brown or gray to colorless. Less often blue, green, black, translucent white, pink, violet, orange, purple and red. |
| Crystal habit |
Octahedral |
| Crystal system |
Isometric-Hexoctahedral (Cubic) |
| Cleavage |
111 (perfect in four directions) |
| Fracture |
Conchoidal(shell-like) |
| Mohs Scale hardness |
10 |
| Luster |
Adamantine |
| Streak |
White |
| Specific gravity |
3.52±0.01 |
| Density |
3.5–3.53 g/cm3 |
| Polish luster |
Adamantine |
| Optical properties |
Singly Refractive |
| Refractive index |
2.4175–2.4178 |
| Birefringence |
None |
| Pleochroism |
None |
| Dispersion |
0.044 |
History
The name diamond is derived from the ancient Greekἀδάμας(adámas), “proper”, “unalterable”, “unbreakable, untamed”, from ἀ- (a-), “un-” + δαμάω (damáō), “I overpower, I tame”.[3]However, diamonds are thought to have been first recognized and mined in India, where significant alluvial deposits of the stone could then be found many centuries ago along the riversPenner, Krishna and Godavari. Diamonds have been known in India for at least 3,000 years but most likely 6,000 years.[4]
Diamonds have been treasured as gemstones since their use as religious icons in ancient India. Their usage inengravingtools also dates to early human history.[5][6]Popularity of diamonds has risen since the 19th century because of increased supply, improved cutting and polishing techniques, growth in the world economy, and innovative and successful advertising campaigns.[7]
In 1813, Humphry Davy used a lens to concentrate the rays of the sun on a diamond in an atmosphere of oxygen, and showed that the only product of the combustion was carbon dioxide, proving that diamond is composed of carbon. Later, he showed that in an atmosphere devoid of oxygen, diamond is converted to graphite.[8]
The most familiar usage of diamonds today is as gemstones used for adornment, a usage which dates back into antiquity. The dispersion of white light into spectral colors is the primary gemological characteristic of gem diamonds. In the twentieth century, experts in the field of gemology have developed methods of grading diamonds and other gemstones based on the characteristics most important to their value as a gem. Four characteristics, known informally as the four Cs, are now commonly used as the basic descriptors of diamonds: these are carat, cut, color, andclarity.[9]
Material properties

Diamond and graphite are twoallotropes of carbon: pure forms of the same element that differ in structure.
A diamond is a transparent crystal of tetrahedrally bonded carbon atoms (sp3) that crystallizes into the diamond latticewhich is a variation of the face centered cubicstructure. Diamonds have been adapted for many uses because of the material’s exceptional physical characteristics. Most notable are its extreme hardness andthermal conductivity(900–2,320 W·m−1·K−1),[10] as well as wide bandgap and high optical dispersion.[11] Above 1,700 °C(1,973 K / 3,583 °F) invacuum or oxygen-free atmosphere, diamond converts to graphite; in air, transformation starts at ~700 °C.[12] Naturally occurring diamonds have a density ranging from 3.15–3.53 g/cm3, with very pure diamond typically extremely close to 3.52 g/cm3.[1]
Hardness
Diamond is the hardest natural material known, where hardness is defined as resistance to scratching and is graded between 1 (softest) and 10 (hardest) using the Mohs scale of mineral hardness. Diamond has a hardness of 10 (hardest) on this scale.[13] Diamond’s hardness has been known since antiquity, and is the source of its name.
The diamond hardness depends on its purity, crystalline perfection and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice).[14] Therefore, whereas it might be possible to scratch some diamonds with other materials, such as boron nitride, the hardest diamonds can only be scratched by other diamonds. In particular,nanocrystalline diamond aggregates were measured to be harder than any large single crystal diamond. Those aggregates are produced by high-pressure high-temperature treatment of graphite or fullerite (C60).[15]
The hardness of diamonds contributes to its suitability as a gemstone. Because it can only be scratched by other diamonds, it maintains its polish extremely well. Unlike many other gems, it is well-suited to daily wear because of its resistance to scratching—perhaps contributing to its popularity as the preferred gem in engagement or wedding rings, which are often worn every day.
The hardest natural diamonds mostly originate from the Copeton and Bingara fields located in the New England area inNew South Wales, Australia. These diamonds are generally small, perfect to semiperfect octahedra, and are used to polish other diamonds. Their hardness is associated with thecrystal growth form, which is single-stage crystal growth. Most other diamonds show more evidence of multiple growth stages, which produce inclusions, flaws, and defect planes in the crystal lattice, all of which affect their hardness. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges.[16]
Industrial use of diamonds has historically been associated with their hardness; this property makes diamond the ideal material for cutting and grinding tools. As the hardest known naturally occurring material, diamond can be used to polish, cut, or wear away any material, including other diamonds. Common industrial adaptations of this ability include diamond-tipped drill bits and saws, and the use of diamond powder as an abrasive. Less expensive industrial-grade diamonds, known as bort, with more flaws and poorer color than gems, are used for such purposes.[17]
Diamond is not suitable for machining ferrous alloys at high speeds as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools when compared to alternatives.[18]
Electrical conductivity
Other specialized applications also exist or are being developed, including use as semiconductors: some blue diamonds are natural semiconductors, in contrast to most other diamonds, which are excellent electricalinsulators.[19]The conductivity and blue color originate from the boron impurity. Boron substitutes for carbon atoms in the diamond lattice, donating a hole into the valence band.[19]
Substantial conductivity is commonly observed in nominally undoped diamond grown by chemical vapor deposition. This conductivity is associated with hydrogen-related species adsorbed at the surface, and it can be removed by annealing or other surface treatments.[20][21]
Toughness
Toughness relates to a material’s ability to resist breakage from forceful impact. The toughness of natural diamond has been measured as 2.0 MPa·m1/2,[22] and the critical stress intensity factor is 3.4 MN·m−3/2.[23] Those values are good compared to other gemstones, but poor compared to most engineering materials. As with any material, the macroscopic geometry of a diamond contributes to its resistance to breakage. Diamond has a cleavage plane and is therefore more fragile in some orientations than others. Diamond cutters use this attribute to cleave some stones, prior to faceting.[24]
Color
Diamond has a wide bandgap of 5.5 eV (or 225 nm) meaning that pure diamond should transmit visible light and appear as a clear colorless crystal. Colors in diamond originate from lattice defects and impurities. The diamond crystal lattice is exceptionally strong and only atoms of nitrogen, boron and hydrogen can be introduced into diamond during the growth at significant concentrations (up to atomic percents). Transition metals Ni and Co, which are commonly used for growth of synthetic diamond by the high-pressure high-temperature techniques, have been detected in diamond as individual atoms, however the maximum concentration is 0.01% for Ni[25] and even much less for Co. Note however, that virtually any element can be introduced in diamond by ion implantation.[26]
Nitrogen is by far the most common impurity found in gem diamonds. Nitrogen is responsible for the yellow and brown in diamonds. Boron is responsible for the gray blue colors.[11]Color in diamond has two additional sources: irradiation (usually by alpha particles), that causes the color in green diamonds; and physical deformation of the diamond crystal known as plastic deformation. Plastic deformation is the cause of color in some brown[27] and perhaps pink and red diamonds.[28] In order of rarity, colorless diamond, by far the most common, is followed by yellow and brown, by far the most common colors, then by blue, green, black, translucent white, pink, violet, orange, purple, and the rarest, red.[24]“Black,” or Carbonado, diamonds are not truly black, but rather contain numerous dark inclusions that give the gems their dark appearance. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice, known as a carbon flaw. The most common impurity, nitrogen, causes a slight to intense yellow coloration depending upon the type and concentration of nitrogen present.[24] The Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from ‘D’ (colorless) to ‘Z’ (light yellow). Diamonds of a different color, such as blue, are called fancy coloreddiamonds, and fall under a different grading scale.[24]
In 2008, the Wittelsbach Diamond, a 35.56 carats (7.11 g) blue diamond once belonging to the King of Spain, fetched over US$24 million at a Christie’s auction.[29] In 2009 a 7.03 carats (1.41 g) blue diamond fetched the highest price per-carat ever paid for a diamond when it was sold at auction for 10.5 million Swiss francs (6.97 million Euro or US$9.5 million at the time) which is in excess of US$1.3 million per carat.[30]
Identification
Diamonds can be identified by their high thermal conductivity. Their high refractive index is also indicative, but other materials have similar refractivity. Diamonds do cut glass, but this does not positively identify a diamond because other materials, such as quartz, also lie above glass on the Mohs scale and can also cut glass. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Hardness tests are infrequently used in practical gemmology because of their potentially destructive nature. The extreme hardness and high value of diamond means that gems are typically polished slowly using painstaking traditional techniques and greater attention to detail than is the case with most other gemstones; these tend to result in extremely flat, highly-polished facets with exceptionally sharp facet edges. Diamonds also possess an extremely high refractive index and fairly high dispersion. Taken together, these factors affect the overall appearance of a polished diamond and most diamantaires still rely upon skilled use of the loupe (magnifying glass) to identify diamonds ‘by eye’.
Natural history
The formation of natural diamond requires very specific conditions—exposure of carbon-bearing materials to highpressure, ranging approximately between 45 and 60kilobars, but at a comparatively low temperature range between approximately 1650–2370 °F (900–1300 °C). These conditions are met in two places on Earth; in the lithospheric mantlebelow relatively stable continental plates, and at the site of ameteorite strike.[31]
Formation in cratons
The conditions for diamond formation to happen in the lithospheric mantle occur at considerable depth corresponding to the aforementioned requirements of temperature and pressure. These depths are estimated between 140 and 190 km though occasionally diamonds have crystallized at depths of 300–400 km as well.[32] The rate at which temperature changes with increasing depth into the Earth varies greatly in different parts of the Earth. In particular, under oceanic plates the temperature rises more quickly with depth, beyond the range required for diamond formation at the depth required. The correct combination of temperature and pressure is only found in the thick, ancient, and stable parts ofcontinental plates where regions of lithosphere known ascratons exist. Long residence in the cratonic lithosphere allows diamond crystals to grow larger.[33]

The slightly misshapen octahedral shape of this rough diamond crystal in matrix is typical of the mineral. Its lustrous faces also indicate that this crystal is from a primary deposit.
Through studies of carbon isotope ratios (similar to the methodology used in carbon dating, except with the stable isotopes C-12 and C-13), it has been shown that the carbon found in diamonds comes from both inorganic and organic sources. Some diamonds, known as harzburgitic, are formed from inorganic carbon originally found deep in the Earth’smantle. In contrast, eclogitic diamonds contain organic carbon from organic detritus that has been pushed down from the surface of the Earth’s crust through subduction (see plate tectonics) before transforming into diamond. These two different source of carbons have measurably different 13C:12C ratios. Diamonds that have come to the Earth’s surface are generally quite old, ranging from under 1 billion to 3.3 billion years old. This is 22% to 73% of the age of the Earth.[33]
Diamonds occur most often as euhedral or roundedoctahedraand twinned octahedra known as macles ormaccles. As diamond’s crystal structure has a cubic arrangement of the atoms, they have many facets that belong to a cube,octahedron, rhombicosidodecahedron, tetrakis hexahedronor disdyakis dodecahedron. The crystals can have rounded off and unexpressive edges and can be elongated. Sometimes they are found grown together or form double “twinned” crystals at the surfaces of the octahedron. These different shapes and habits of the diamonds result from differing external circumstances. Diamonds (especially those with rounded crystal faces) are commonly found coated in nyf, an opaque gum-like skin.[34]
Formation in meteorite impact craters
Diamonds can also form in other natural high-pressure events. Very small diamonds, known as microdiamondsornanodiamonds, have been found in meteorite impact craters. Such impact events create shock zones of high pressure and temperature suitable for diamond formation. Impact-type microdiamonds can be used as an indicator of ancient impact craters.[31]
Extraterrestrial formation
Not all diamonds found on Earth originated here. A type of diamond called carbonado diamond that is found in South America and Africa may have been deposited there via an asteroid impact (not formed from the impact) about 3 billion years ago. These diamonds may have formed in the intrastellar environment, but as of 2008, there was no scientific consensus on how carbonado diamonds originated.[35][36]
Presolar grains in many meteorites found on Earth contain nanodiamonds of extraterrestrial origin, probably formed insupernovas. Scientific evidence indicates that white dwarfstars have a core of crystallized carbon and oxygen nuclei. The largest of these found in the universe so far, BPM 37093, is located 50 light-years (4.7×1014 km) away in theconstellation Centaurus. A news release from the Harvard-Smithsonian Center for Astrophysics described the 2,500-mile (4,000 km) wide stellar core as a diamond.[37] It was referred to as Lucy, after the Beatles song “Lucy in the Sky With Diamonds”.[16][38]
Surfacing
Diamond-bearing rock is brought close to the surface through deep-origin volcanic eruptions. The magma for such a volcano must originate at a depth where diamonds can be formed[33]—150 km (93 mi) or more (three times or more the depth of source magma for most volcanoes). This is a relatively rare occurrence. These typically small surface volcanic craters extend downward in formations known asvolcanic pipes.[33] The pipes contain material that was transported toward the surface by volcanic action, but was not ejected before the volcanic activity ceased. During eruption these pipes are open to the surface, resulting in open circulation; many xenoliths of surface rock and even wood and/or fossils are found in volcanic pipes. Diamond-bearing volcanic pipes are closely related to the oldest, coolest regions of continental crust (cratons). This is because cratons are very thick, and their lithospheric mantle extends to great enough depth that diamonds are stable. Not all pipes contain diamonds, and even fewer contain enough diamonds to make mining economically viable.[33]
The magma in volcanic pipes is usually one of two characteristic types, which cool into igneous rock known as either kimberlite or lamproite.[33] The magma itself does not contain diamond; instead, it acts as an elevator that carries deep-formed rocks (xenoliths), minerals (xenocrysts), and fluids upward. These rocks are characteristically rich inmagnesium-bearing olivine, pyroxene, andamphiboleminerals[33] which are often altered to serpentineby heat and fluids during and after eruption. Certain indicator mineralstypically occur within diamantiferous kimberlites and are used as mineralogical tracers by prospectors, who follow the indicator trail back to the volcanic pipe which may contain diamonds. These minerals are rich in chromium (Cr) ortitanium (Ti), elements which impart bright colors to the minerals. The most common indicator minerals are chromiangarnets (usually bright red Cr-pyrope, and occasionally green ugrandite-series garnets), eclogitic garnets, orange Ti-pyrope, red high-Cr spinels, dark chromite, bright green Cr-diopside, glassy green olivine, blackpicroilmenite, and magnetite. Kimberlite deposits are known as blue ground for the deeper serpentinized part of the deposits, or as yellow ground for the near surface smectiteclay and carbonate weathered andoxidized portion.[33]
Once diamonds have been transported to the surface by magma in a volcanic pipe, they may erode out and be distributed over a large area. A volcanic pipe containing diamonds is known as a primary source of diamonds.Secondary sources of diamonds include all areas where a significant number of diamonds, eroded out of their kimberlite or lamproite matrix, and accumulated because of water or wind action. These include alluvial deposits and deposits along existing and ancient shorelines, where loose diamonds tend to accumulate because of their approximate size and density. Diamonds have also rarely been found in deposits left behind by glaciers (notably in Wisconsin andIndiana); however, in contrast to alluvial deposits, glacial deposits are minor and are therefore not viable commercial sources of diamond.[33]
Commercial markets
The diamond industry can be broadly separated into two basically distinct categories: one dealing with gem-grade diamonds and another for industrial-grade diamonds. While a large trade in both types of diamonds exists, the two markets act in dramatically different ways.
Gemstones
A large trade in gem-grade diamonds exists. Unlike precious metals such as gold or platinum, gem diamonds do not trade as a commodity: there is a substantial mark-up in the retail sale of diamonds. Contrary to popular belief, there is a well-established market for resale of polished diamonds (e.g. pawnbroking, auctions, second-hand jewelry stores, diamantaires, bourses, etc.). One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations. 92% of diamond pieces cut in 2003 were inSurat,Gujarat, India.[39] Other important centers of diamond cutting and trading are Antwerp, where the International Gemological Institute is based, London, New York, Tel Aviv, and Amsterdam. A single company—De Beers—controls a significant proportion of the trade in diamonds. They are based in Johannesburg, South Africa and London, England. One contributory factor is the geological nature of diamond deposits: several large primary kimberlite-pipe mines each account for significant portions of market share (such as theJwaneng mine in Botswana, which is a single large pit operated by De Beers that can produce between 12.5 to 15 million carats of diamonds per year[40]), whereas secondary alluvial diamond deposits tend to be fragmented amongst many different operators because they can be dispersed over many hundreds of square kilometers (e.g., alluvial deposits in Brazil).
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers. The most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled.[41] This makes Antwerp the de facto ‘world diamond capital’. New York, however, along with the rest of the United States, is where almost 80% of the world’s diamonds are sold, including auction sales. Also, the largest and most unusually shaped rough diamonds end up in New York.[41] The De Beers company, as the world’s largest diamond miner holds a clearly dominant position in the industry, and has done so since soon after its founding in 1888 by the British imperialistCecil Rhodes. De Beers owns or controls a significant portion of the world’s rough diamond production facilities (mines) anddistribution channels for gem-quality diamonds. The company and its subsidiaries own mines that produce some 40 percent of annual world diamond production. At one time it was thought over 80 percent of the world’s rough diamonds passed through the Diamond Trading Company (DTC, a subsidiary of De Beers) in London,[42] but presently the figure is estimated at around 40 percent.[43] De Beers sold off the vast majority its diamond stockpile in the late 1990s – early 2000s[44] and the remainder largely represents working stock (diamonds that are being sorted before sale).[45] This was well documented in the press[46] but remains little known to the general public.
The De Beers diamond advertising campaign is acknowledged as one of the most successful and innovative campaigns in history. N. W. Ayer & Son, the advertising firm retained by De Beers in the mid-20th century, succeeded in reviving the American diamond market and opened up new markets, even in countries where no diamond tradition had existed before. N. W. Ayer’s multifaceted marketing campaign included product placement, advertising the diamond itself rather than the De Beers brand, and building associations with celebrities and royalty. This coordinated campaign has lasted decades and continues today; it is perhaps best captured by the slogan “a diamond is forever”.[7]
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. The WFDB consists of independent diamond bourses in major cutting centers such as Tel Aviv, Antwerp, Johannesburg and other cities across the USA, Europe and Asia.
In 2000, the WFDB and The International Diamond Manufacturers Association established the World Diamond Council to prevent the trading of diamonds used to fund war and inhumane acts. WFDB’s additional activities also include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Industrial grade

A scalpel with synthetic diamond blade
The market for industrial-grade diamonds operates much differently from its gem-grade counterpart. Industrial diamonds are valued mostly for their hardness and heat conductivity, making many of the gemological characteristics of diamonds, such as clarity and color, irrelevant for most applications. This helps explain why 80% of mined diamonds (equal to about 135 million carats or 27 metric tons annually), unsuitable for use as gemstones, are destined for industrial use. In addition to mined diamonds, synthetic diamonds found industrial applications almost immediately after their invention in the 1950s; another 570 million carats (114 tons) of synthetic diamond is produced annually for industrial use. Approximately 90% of diamond grinding grit is currently of synthetic origin.[47]
The dominant industrial use of diamond is in cutting, drilling, grinding, and polishing. Most uses of diamonds in these technologies do not require large diamonds; in fact, most diamonds that are gem-quality except for their small size, can find an industrial use. Diamonds are embedded in drill tips or saw blades, or ground into a powder for use in grinding and polishing applications. Specialized applications include use in laboratories as containment for high pressure experiments(see diamond anvil cell), high-performancebearings, and limited use in specialized windows.[48]
With the continuing advances being made in the production of synthetic diamonds, future applications are becoming feasible. Garnering much excitement is the possible use of diamond as a semiconductor suitable to buildmicrochipsfrom, or the use of diamond as a heat sink[49] inelectronics.
The boundary between gem-quality diamonds and industrial diamonds is poorly defined and partly depends on market conditions (for example, if demand for polished diamonds is high, some suitable stones will be polished into low-quality or small gemstones rather than being sold for industrial use). Within the category of industrial diamonds, there is a sub-category comprising the lowest-quality, mostly opaque stones, which are known as bort or ‘boart’.[48]
Supply chain
Approximately 130 million carats (26,000 kg (57,000 lb)) are mined annually, with a total value of nearly USD $9 billion, and about 100,000 kg (220,000 lb) are synthesized annually.[50]
Roughly 49% of diamonds originate from central and southern Africa, although significant sources of the mineral have been discovered in Canada, India, Russia, Brazil, and Australia. They are mined from kimberlite and lamproite volcanic pipes, which can bring diamond crystals, originating from deep within the Earth where high pressures and temperatures enable them to form, to the surface. The mining and distribution of natural diamonds are subjects of frequent controversy such as with concerns over the sale of conflict diamonds or blood diamonds by African paramilitary groups.[51] The diamond supply chain is controlled by a limited number of powerful businesses, and is also highly concentrated in a small number of locations around the world (see figure).
Mining, sources and production
Only a very small fraction of the diamond ore consists of actual diamonds. The ore is crushed, during which care is required not to destroy larger diamonds, and then sorted by density. Today, diamonds are located in the diamond-rich density fraction with the help of X-ray fluorescence, after which the final sorting steps are done by hand. Before the use of X-rays became commonplace, the separation was done with grease belts; diamonds have a stronger tendency to stick to grease than the other minerals in the ore.[24]
Historically diamonds were found only in alluvial deposits insouthern India.[52] India led the world in diamond production from the time of their discovery in approximately the 9th century BC[4][53] to the mid-18th century AD, but the commercial potential of these sources had been exhausted by the late 18th century and at that time India was eclipsed by Brazil where the first non-Indian diamonds were found in 1725.[4]
Diamond production of primary deposits (kimberlites and lamproites) only started in the 1870s after the discovery of the Diamond fields in South Africa.[54] Production has increased over time and now an accumulated total of 4.5 billion carats have been mined since that date.[55] Interestingly 20% of that amount has been mined in the last 5 years alone and during the last ten years 9 new mines have started production while 4 more are waiting to be opened soon. Most of these mines are located in Canada, Zimbabwe, Angola, and one in Russia.[55]
In the U.S., diamonds have been found in Arkansas, Colorado, and Montana.[56][57] In 2004, a startling discovery of a microscopic diamond in the U.S. led to the January 2008 bulk-sampling of kimberlite pipes in a remote part ofMontana.[57][58]
Today, most commercially viable diamond deposits are inRussia (mostly in Yakutia, for example Mir pipeandUdachnaya pipe), Botswana, Australia (Northern and Western Australia) and the Democratic Republic of Congo.[59]
In 2005, Russia produced almost one-fifth of the global diamond output, reports the British Geological Survey. Australia boasts the richest diamantiferous pipe with production reaching peak levels of 42 metric tons (41 LT; 46 ST) per year in the 1990s.[56]
There are also commercial deposits being actively mined in the Northwest Territories of Canada and Brazil. Diamond prospectors continue to search the globe for diamond-bearing kimberlite and lamproite pipes.
Controversial sources
In some of the more politically unstable central African and west African countries, revolutionary groups have taken control of diamond mines, using proceeds from diamond sales to finance their operations. Diamonds sold through this process are known as conflict diamonds or blood diamonds.[51] Major diamond trading corporations continue to fund and fuel these conflicts by doing business with armed groups. In response to public concerns that their diamond purchases were contributing to war and human rights abusesin central and western Africa, the United Nations, the diamond industry and diamond-trading nations introduced theKimberley Process in 2002. The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. This is done by requiring diamond-producing countries to provide proof that the money they make from selling the diamonds is not used to fund criminal or revolutionary activities. Although the Kimberley Process has been moderately successful in limiting the number of conflict diamonds entering the market, some still find their way in. 2–3% of all diamonds traded today are potentially conflict diamonds.[60] Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered “clean”.[61]
The Canadian Government has set up a body known as Canadian Diamond Code of Conduct[62] to help authenticate Canadian diamonds. This is a very stringent tracking system of diamonds and helps protect the ‘conflict free’ label of Canadian diamonds.[63]
Distribution
The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines (it withdrew from purchasing diamonds on the open market in 1999 and ceased purchasing Russian diamonds mined by Russian company Alrosa, at the end of 2008. Alrosa has successfully appealed against a European court ruling[64], but is not reported to have resumed sales to De Beers, although it sells its diamond production to other traders.[65]).
Once purchased by Sightholders (which is a trademark term referring to the companies that have a three-year supply contract with DTC), diamonds are cut and polished in preparation for sale as gemstones (‘industrial’ stones are regarded as a by-product of the gemstone market; they are used for abrasives). The cutting and polishing of rough diamonds is a specialized skill that is concentrated in a limited number of locations worldwide. Traditional diamond cutting centers are Antwerp, Amsterdam, Johannesburg, New York, and Tel Aviv. Recently, diamond cutting centers have been established in China, India, Thailand, Namibia and Botswana. Cutting centers with lower cost of labor, notablySurat in Gujarat, India, handle a larger number of smaller carat diamonds, while smaller quantities of larger or more valuable diamonds are more likely to be handled in Europe or North America. The recent expansion of this industry in India, employing low cost labor, has allowed smaller diamonds to be prepared as gems in greater quantities than was previously economically feasible.[41]
Diamonds which have been prepared as gemstones are sold on diamond exchanges called bourses.. There are 26 registered diamond bourses in the world.[66] Bourses are the final tightly controlled step in the diamond supply chain; wholesalers and even retailers are able to buy relatively small lots of diamonds at the bourses, after which they are prepared for final sale to the consumer. Diamonds can be sold already set in jewelry, or sold unset (“loose”). According to the Rio Tinto Group, in 2002 the diamonds produced and released to the market were valued at US$9 billion as rough diamonds, US$14 billion after being cut and polished, US$28 billion in wholesale diamond jewelry, and US$57 billion in retail sales.[67]
Synthetics, simulants, and enhancements
Synthetics

Synthetic diamonds of various colors grown by the high-pressure high-temperature technique
Synthetic diamonds are diamond crystals that are manufactured in a laboratory, as opposed to natural diamonds which form naturally within the Earth. The gemological and industrial uses of diamond have created a large demand for rough stones. This demand has long been satisfied in large part by synthetic diamonds, which have been manufactured by various processes for more than half a century. However, in recent years it has become possible to produce gem-quality synthetic diamonds of significant size.[33]
The majority of commercially available synthetic diamonds are yellow in color and produced by so called High Pressure High Temperature (HPHT) processes.[68] The yellow color is caused by nitrogen impurities. Other colors may also be reproduced such as blue, green or pink, which are a result of the addition of boron or from irradiation after synthesis.[69]

Colorless gem cut from diamond grown by chemical vapor deposition
Another popular method of growing synthetic diamond is chemical vapor deposition (CVD). The growth occurs under low pressure (below atmospheric pressure). It involves feeding a mixture of gases (typically 1 to 99 methanetohydrogen) into a chamber and splitting them to chemically active radicals in a plasma ignited by microwaves, hot filament, arc discharge, welding torch or laser.[70] This method is mostly used for coatings, but can also produce single crystals several millimeters in size (see picture).[50]
At present, the annual production of gem quality synthetic diamonds is only a few thousand carats, whereas the total production of natural diamonds is around 120 million carats. Despite this fact, a purchaser is more likely to encounter a synthetic when looking for a fancy-colored diamond because nearly all synthetic diamonds are fancy-colored, while only 0.01% of natural diamonds are fancy-colored.[71] Producing large synthetic diamonds threatens the business model of the diamond industry. The ultimate effect of the ready availability of gem-quality diamonds at low cost in the future is hard to predict.
Simulants

Gem-cut synthetic silicon carbide
A diamond simulant is defined as a non-diamond material that is used to simulate the appearance of a diamond. Diamond-simulant gems are often referred to as diamante. The most familiar diamond simulant to most consumers is cubic zirconia. The popular gemstone moissanite (silicon carbide) is often treated as a diamond simulant, although it is a gemstone in its own right. While moissanite does look similar to diamond, its main disadvantage as a diamond simulant is that cubic zirconia is far cheaper and arguably equally convincing. Both cubic zirconia and moissanite are produced synthetically.[72]
Enhancements
Diamond enhancements are specific treatments performed on natural or synthetic diamonds (usually those already cut and polished into a gem), which are designed to better the gemological characteristics of the stone in one or more ways. These include laser drilling to remove inclusions, application of sealants to fill cracks, treatments to improve a white diamond’s color grade, and treatments to give fancy color to a white diamond.
Coatings are increasingly used to give a diamond simulant such as cubic zirconia a more “diamond-like” appearance. One such substance is diamond-like carbon—an amorphous carbonaceous material that has some physical properties similar to those of the diamond. Advertising suggests that such a coating would transfer some of these diamond-like properties to the coated stone, hence enhancing the diamond simulant. However, modern techniques such as Raman Spectroscopy should easily identify such as treatment.[73]
Identification
It was stated that annealing can convert typically brown synthetically made (CVD) diamonds into colorless diamonds, and that after having sent these diamonds for diamond jewelry identification, they were not identified as different from natural diamonds.[74] Such claims are often made for new synthetics, simulants, and treated stones, so it is important to validate how the stones were submitted for identification.
Properly trained and equipped gemologists can distinguish between natural diamonds and synthetic diamonds. They can also identify the vast majority of treated natural diamonds, two exceptions being a small minority of HPHT-treated Type II diamonds and some artificially irradiated green diamonds. “Perfect” crystals (at the atomic lattice level) have never been found, so both natural and synthetic diamonds always possess characteristic imperfections, arising from the circumstances of their crystal growth, that allow them to be distinguished from each other.[75]
Laboratories use techniques such as spectroscopy, microscopy and luminescence under shortwave ultraviolet light to determine a diamond’s origin. They also use specially made machines to aid them in the identification process. Two screening machines are the DiamondSure and theDiamondView, both produced by the DTC and marketed by theGIA.[76]
Several methods for identifying synthetic diamonds can be performed, depending on the method of production and the color of the diamond. CVD diamonds can usually be identified by an orange fluorescence. D-J colored diamonds can be screened through the Swiss Gemmological Institute‘s[77]Diamond Spotter. Stones in the D-Z color range can be examined through the DiamondSure UV/visible spectrometer, a tool developed by De Beers.[75] Similarly, natural diamonds usually have minor imperfections and flaws, such as inclusions of foreign material, that are not seen in synthetic diamonds. |
|
Aquamarine and maxixe
Aquamarine (from Lat. aqua marina, “water of the sea”) is a blue or turquoisevariety of beryl. It occurs at most localities which yield ordinary beryl, some of the finest coming from Russia. The gem-gravel placer deposits of Sri Lanka contain aquamarine. Clear yellow beryl, such as occurs in Brazil, is sometimes called aquamarine chrysolite. When corundum presents the bluish tint of typical aquamarine, it is often termed Oriental aquamarine. The deep blue version of aquamarine is called maxixe. Its color fades to white when exposed to sunlight or is subjected to heat treatment, though the color returns with irradiation.
The pale blue color of aquamarine is attributed to Fe2+. The Fe3+ ions produce golden-yellow color, and when both Fe2+ and Fe3+ are present, the color is a darker blue as in maxixe. Decoloration of maxixe by light or heat thus may be due to the charge transfer Fe3+ and Fe2+.[4][12][13][14] Dark-blue maxixe color can be produced in green, pink or yellow beryl by irradiating it with high-energy particles (gamma rays, neutrons or even X-rays).[15]
In the United States, aquamarines can be found at the summit of Mt. Antero in the Sawatch Range in central Colorado. In Wyoming, aquamarine has been discovered in the Big Horn mountains, near Powder River Pass. In Brazil, there are mines in the states ofMinas Gerais,Espírito Santo and Bahia. The Mines of Colombia, Zambia, Madagascar, Malawi, Tanzaniaand Kenya also produce aquamarine.
The biggest aquamarine ever mined was found at the city of Marambaia, Minas Gerais, Brazil, in 1910. It weighed over 110 kg, and its dimensions were 48.5 cm long and 42 cm in diameter.
Culture usage
Emerald
Emerald refers to green beryl, colored by trace amounts of chromium and sometimes vanadium.[4][16] The word “emerald” comes from Latinsmaragdus, its original source being a Semitic word izmargad or theSanskrit word,marakata, meaning “green”.[17] Most emeralds are highlyincluded, so their brittleness (resistance to breakage) is classified as generally poor.
Emeralds in antiquity were mined by the Egyptians and in Austria, as well as Swat in northernPakistan.[18] A rare type of emerald known as a trapiche emerald is occasionally found in the mines of Colombia. A trapiche emerald exhibits a “star” pattern; it has raylike spokes of dark carbon impurities that give the emerald a six-pointed radial pattern. It is named for thetrapiche, a grinding wheel used to process sugarcane in the region. Colombian emeralds are generally the most prized due to their transparency and fire. Some of the most rare emeralds come from three main emerald mining areas in Colombia: Muzo, Coscuez, and Chivor. Fine emeralds are also found in other countries, such as Zambia, Brazil, Zimbabwe, Madagascar,Pakistan, India,Afghanistan and Russia. In the US, emeralds can be found in Hiddenite, North Carolina. In 1998, emeralds were discovered in theYukon.
Emerald is a rare and valuable gemstone and, as such, it has provided the incentive for developing synthetic emeralds. Both hydrothermal[19] and flux-growth synthetics have been produced. The first commercially successful emerald synthesis process was that of Carroll Chatham. The other large producer of flux emeralds was Pierre Gilson Sr., which has been on the market since 1964. Gilson’s emeralds are usually grown on natural colorless beryl seeds which become coated on both sides. Growth occurs at the rate of 1 mm per month, a typical seven-month growth run producing emerald crystals of 7 mm of thickness.[20] The green color of emeralds is attributed to presense of Fe3+ and Fe2+ ions.[12] [13][14]
Goshenite

Crystal structure of beryl
Colorless beryl is called goshenite. The name originates from Goshen, Massachusetts where it was originally described. Since all these color varieties are caused by impurities and pure beryl is colorless, it might be tempting to assume that goshenite is the purest variety of beryl. However, there are several elements that can act as inhibitors to color in beryl and so this assumption may not always be true. The name goshenite has been said to be on its way to extinction and yet it is still commonly used in the gemstone markets. Goshenite is found to some extent in almost all beryl localities. In the past, goshenite was used for manufacturing eyeglasses and lenses owing to its transparency. Nowadays, it is most commonly used for gemstone purposes and also considered as a source of beryllium. [21][22]
The gem value of gohenite is relatively low. However, goshenite can be colored yellow, green, pink, blue and in intermediate colors by irradiating it with high-energy particles. The resoluting color depends on the content of Ca, Sc, Ti, V, Fe, and Co inmpurities.[12]
Golden beryl and heliodor
Golden beryl can range in colors from pale yellow to a brilliant gold. Unlike emerald, golden beryl has very few flaws. The term “golden beryl” is sometimes synonymous with heliodor(from Greek helios “sun”), but golden beryl refers to pure yellow or golden yellow shades, while heliodor refers to the greenish-yellow shades. The golden yellow color is attributed to Fe3+ ions.[4][12] Both golden beryl and heliodor are used as gems. Probably the largest cut golden beryl is the flawless 2054 carat stone on display on display in the Hall of Gems,Washington, D.C.[23] |
Amethyst
Amethyst is a violet variety of quartz often used in jewelry. The name comes from the Ancient Greek a- (“not”) and methustos(“intoxicated”), a reference to the belief that the stone protected its owner from drunkenness; the ancient Greeks and Romans wore amethyst and made drinking vessels of it in the belief that it would prevent intoxication.
Chemistry
Amethyst is the violet variety of quartz; its chemical formula is SiO2.
In the 20th century, the color of amethyst was attributed to the presence of manganese. However, since it is capable of being greatly altered and even discharged by heat, the color was believed by some authorities to be from an organic source. Ferric thiocyanatewas suggested, and sulfur was said to have been detected in the mineral.
More recent work has shown that amethysts’ coloration is due to ferric iron impurities.[1]Further study has shown a complex interplay of iron and aluminium is responsible for the color.[2]
On exposure to heat, amethyst generally becomes yellow, and much of the citrine, cairngorm, or yellow quartz of jewelry is said to be merely “burnt amethyst”. Veins of amethystine quartz are apt to lose their color on the exposed outcrop[citation needed].
Synthetic amethyst is made to imitate the best quality amethyst. Its chemical and physical properties are so similar to that of natural amethyst that it can not be differentiated with absolute certainty without advanced gemological testing (which is often cost-prohibitive). There is one test based on “Brazil law twinning” (a form of quartz twinning where right and left hand quartz structures are combined in a single crystal[3]) which can be used to identify synthetic amethyst rather easily. In theory however it is possible to create this material synthetically as well, but this type is not available in large quantities in the market.[4]
Composition
Amethyst is composed of an irregular superposition of alternate lamellae of right-handed and left-handed quartz. It has been shown that this structure may be due to mechanical stresses.
Because it has a hardness of seven on the Mohs scale, amethyst is suitable for use in jewelery.
Hue and tone
Amethyst occurs in primary hues from a light pinkish violet to a deep purple. Amethyst may exhibit one or both secondary hues, red and blue. The ideal grade is called “Deep Siberian” and has a primary purple hue of around 75–80 percent, 15–20 percent blue and (depending on the light source) red secondary hues.[4]

The inside of an AmethystGeode.
History
Amethyst was used as a gemstone by the ancient Egyptians and was largely employed in antiquity for intaglios. The Greeks believed amethyst gems could prevent intoxication, while medieval European soldiers wore amethyst amulets as protection in battle..[citation needed]Beads of amethyst were found in Anglo-Saxon graves in England.[citation needed]
A huge geode, or “amethyst-grotto”, from near Santa Cruz in southern Brazil was exhibited at the Düsseldorf, Germany Exhibition of 1902.
Mythology
The Greek word “amethystos” may be translated as “not drunken”. Amethyst was considered to be a strong antidote against drunkenness, which is why wine goblets were often carved from it. In Greek mythology, Dionysus, the god of intoxication, was pursuing a maiden named Amethystos, who refused his affections.. Amethystos prayed to the gods to remain chaste, which the goddess Artemis granted and transformed her into a white stone. Humbled by Amethystos’s desire to remain chaste, Dionysus poured wine over the stone as an offering, dyeing the crystals purple.
Variations of the story include that Dionysus had been insulted by a mortal and swore to slay the next mortal who crossed his path, creating fierce tigers to carry out his wrath.. The mortal turned out to be a beautiful young woman, Amethystos, who was on her way to pay tribute to Artemis. Her life is spared by Artemis, who transformed the maiden into a statue of pure crystalline quartz to protect her from the brutal claws. Dionysus wept tears of wine in remorse for his action at the sight of the beautiful statue. The god’s tears then stained the quartz purple.[5] Another variation involves the goddess Rhea presenting Dionysus with the amethyst stone to preserve the wine-drinker’s sanity.[6]
Geographic distribution
Amethyst is produced in abundance from the state of Minas Gerais in Brazil where it occurs in large geodes within volcanic rocks. It is also found and mined in South Korea. The largest opencast amethyst vein in the world is in Maissau, Lower Austria. Many of the hollow agates of Brazil and Uruguay contain a crop of amethyst crystals in the interior. Much fine amethyst comes from Russia, especially from near Mursinka in the Ekaterinburg district, where it occurs in drusy cavities in granitic rocks. Many localities in Indiayield amethyst. One of the largest global amethyst producers is Zambia with an annual production of about 1,000 t.

Museum-quality piece of Amethyst
Amethyst occurs at many localities in the United States, but these specimens are rarely fine enough for use in jewelry. Among these may be mentioned Amethyst Mountain, Texas;Yellowstone National Park; Delaware County, Pennsylvania; Haywood County, North Carolina; Deer Hill and Stow, Maine. It is found also in the Lake Superior region. Amethyst is relatively common in Ontario, and in various locations throughout Nova Scotia, but uncommon elsewhere in Canada.
Value
Traditionally included in the cardinal, or most valuable, gemstones (along with diamond,sapphire, ruby, and emerald), amethyst has lost much of its value due to the discovery of extensive deposits in locations such as Brazil. The highest grade amethyst (called “Deep Russian”) is exceptionally rare and therefore its value is dependent on the demand of collectors when one is found. It is however still orders of magnitude lower than the highest grade sapphires or rubies (Padparadscha sapphire or “pigeon’s blood” ruby).[4]
|
Garnet
The garnet group includes a group of minerals that have been used since the Bronze Age asgemstones and abrasives. The name “garnet” may come from either the Middle English wordgernet meaning ‘dark red’, or the Latin granatus (“grain“), possibly a reference to the Punica granatum (“pomegranate“), a plant with red seeds similar in shape, size, and color to some garnet crystals.
Six common species of garnet are recognized based on their chemical composition. They arepyrope, almandine, spessartine,grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: 1. pyrope-almandine-spessarite and 2. uvarovite-grossular-andradite.
Physical properties
Properties
Garnets species are found in many colors including red, orange, yellow, green, blue, purple, brown, black, pink and colorless. The rarest of these is the blue garnet, discovered in the late 1990s in Bekily, Madagascar. It is also found in parts of the United States,Russia andTurkey. It changes color from blue-green in the daylight to purple in incandescent light, as a result of the relatively high amounts of vanadium (about 1 wt.% V2O3). Other varieties of color-changing garnets exist.. In daylight, their color ranges from shades of green, beige, brown, gray, and blue, but in incandescent light, they appear a reddish or purplish/pink color. Because of their color changing quality, this kind of garnet is often mistaken for Alexandrite.
Garnet species’s light transmission properties can range from the gemstone-quality transparent specimens to the opaque varieties used for industrial purposes as abrasives. The mineral’s luster is categorized as vitreous (glass-like) or resinous (amber-like).
Crystal structure

Molecular model of garnet.
Garnets are nesosilicates having the general formula X3Y2(SiO4)3. The X site is usually occupied by divalent cations (Ca2+, Mg2+,Fe2+) and the Y site by trivalent cations (Al3+, Fe3+,Cr3+) in an octahedral/tetrahedral framework with [SiO4]4− occupying the tetrahedra.[2]Garnets are most often found in the dodecahedral crystal habit, but are also commonly found in the trapezohedron habit. (Note: the word “trapezohedron” as used here and in most mineral texts refers to the shape called a Deltoidal icositetrahedronin solid geometry.) They crystallize in the cubic system, having three axes that are all of equal length and perpendicular to each other. Garnets do not show cleavage, so when they fracture under stress, sharp irregular pieces are formed.
Hardness
Because the chemical composition of garnet varies, the atomic bonds in some species are stronger than in others. As a result, this mineral group shows a range of hardness on theMohs Scale of about 6.5 to 7.5. The harder species, like almandine, are often used for abrasive purposes.
Garnet group endmember species
Pyralspite garnets – Aluminium in Y site
Almandine

Almandine in metamorphic rock
Almandine, sometimes incorrectly called almandite, is the modern gem known as carbuncle (though originally almost any red gemstone was known by this name). The term “carbuncle” is derived from the Latin meaning “live coal” or burning charcoal. The name Almandine is a corruption of Alabanda, a region in Asia Minor where these stones were cut in ancient times. Chemically, almandine is an iron-aluminium garnet with the formula Fe3Al2(SiO4)3; the deep red transparent stones are often called precious garnet and are used as gemstones (being the most common of the gem garnets). Almandine occurs in metamorphic rocks likemicaschists, associated with minerals such as staurolite, kyanite, andalusite, and others. Almandine has nicknames of Oriental garnet, almandine ruby, and carbuncle.
Pyrope
Pyrope (from the Greek pyrōpós meaning “fire-eyed”) is red in color and chemically a magnesium aluminium silicate with the formula Mg3Al2(SiO4)3, though the magnesium can be replaced in part by calcium and ferrous iron. The color of pyrope varies from deep red to almost black. Transparent pyropes are used as gemstones.
A variety of pyrope from Macon County, North Carolina is a violet-red shade and has been called rhodolite, from the Greek meaning “a rose.” In chemical composition it may be considered as essentially an isomorphous mixture of pyrope and almandine, in the proportion of two parts pyrope to one part almandine. Pyrope has tradenames some of which aremisnomers; Cape ruby, Arizona ruby, California ruby, Rocky Mountain ruby, and Bohemian garnet from the Czech Republic. Another intriguing find is the blue color-changing garnets from Madagascar, a pyrope spessartine mix. The color of these blue garnets is not like sapphire blue in subdued daylight but more reminiscent of the grayish blues and greenish blues sometimes seen in spinel. However, in white LED light the color is equal to the best cornflower blue sapphire, or D block tanzanite; this is due to the blue garnet’s ability to absorb the yellow component of the emitted light.
Pyrope is an indicator mineral for high-pressure rocks. The garnets from mantle derived rocks,peridotites and eclogites, commonly contain a pyrope variety.
Spessartine

Spessartine (the reddish mineral)
Spessartine or spessartite is manganese aluminium garnet, Mn3Al2(SiO4)3. Its name is derived from Spessart in Bavaria. It occurs most often in granite pegmatite and allied rock types and in certain low grade metamorphic phyllites. Spessartine of an orange-yellow is found in Madagascar. Violet-red spessartines are found in rhyolites in Colorado and Maine.
Ugrandite group – calcium in X site
Andradite

Andradite (the black mineral)
Andradite is a calcium-iron garnet, Ca3Fe2(SiO4)3, is of variable composition and may be red, yellow, brown, green or black. The recognized varieties are topazolite (yellow or green),demantoid (green) and melantite (black). Andradite is found both in deep-seatedigneous rockslike syenite as well as serpentines, schists, and crystalline limestone. Demantoid has been called the “emerald of theUrals” from its occurrence there, and is one of the most prized of garnet varieties. Topazolite is a golden yellow variety and melanite is a black variety.
Grossular
Grossular is a calcium-aluminium garnet with the formula Ca3Al2(SiO4)3, though the calcium may in part be replaced by ferrous iron and the aluminium by ferric iron. The name grossular is derived from the botanical name for the gooseberry, grossularia, in reference to the green garnet of this composition that is found in Siberia. Other shades include cinnamon brown (cinnamon stone variety), red, and yellow. Because of its inferior hardness to zircon, which the yellow crystals resemble, they have also been called hessonite from the Greek meaning inferior. Grossular is found in contact metamorphosed limestones with vesuvianite, diopside,wollastonite andwernerite.
One of the most sought after varieties of gem garnet is the fine green grossular garnet from Kenya and Tanzania called tsavorite. This garnet was discovered in the 1960s in the Tsavo area of Kenya, from which the gem takes its name.
Uvarovite
Uvarovite is a calcium chromium garnet with the formula Ca3Cr2(SiO4)3. This is a rather rare garnet, bright green in color, usually found as small crystals associated with chromite inperidotite, serpentinite, and kimberlites. It is found in crystalline marbles andschists in theUral mountains of Russia
Color change garnets
Garnet members of the pyrope-spessartine solid-solution series from Bekily in Madagascar display several colors depending on the light source. The alexandrite-like color change from blue-green in daylight to purple in incandescent light is mainly caused by relatively high amounts of vanadium. Although they look a lot like alexandrites they are different because they change color throughout the day. They are green or blue grey in the early morning and reddish in the late afternoon or in strong sunlight. Bekily garnets will appear red in the afternoon while the alexandrites remain green. Garnets from other parts of East Africa also change color but as they normally change from brown or orange to red, they don’t look much like alexandrite.
Some of the stones are almost blue especially under fluorescent light but most of them are grey blue or green in daylight and change to red under incandescent or late afternoon light. The stones can show an excellent color change and can easily be confused withalexandrite.[3]
Synthetic garnets
In yttrium iron garnet (YIG), Y3Fe2(FeO4)3, the five iron(III) ions occupy two octahedral and three tetrahedral sites, with the yttrium(III) ions coordinated by eight oxygen ions in an irregular cube. The iron ions in the two coordination sites exhibit different spins, resulting inmagnetic behaviour. YIG is a ferrimagnetic material having a Curie temperature of 550 K. By substituting specific sites with rare earth elements, for example, interesting magnetic properties can be obtained.
One example for this is gadolinium gallium garnet, Gd3Ga2(GaO4)3, which is synthesized for use in magnetic bubble memory..
Yttrium aluminium garnet (YAG), Y3Al2(AlO4)3, is used for synthetic gemstone. When doped with neodymium (Nd3+), these YAl-garnets are useful as the lasing medium in lasers.
Geological importance of garnet

Garnet var. Spessartine, Putian City, Putian Prefecture, Fujian Province, China
The Garnet group is a key mineral in interpreting the genesis of many igneous andmetamorphic rocks via geothermobarometry..Diffusion of elements is relatively slow in garnet compared to rates in many other minerals, and garnets are also relatively resistant toalteration. Hence, individual garnets commonly preserve compositional zonations that are used to interpret the temperature-time histories of the rocks in which they grew. Garnet grains that lack compositional zonation commonly are interpreted as having been homogenized by diffusion, and the inferred homogenization also has implications for the temperature-time history of the host rock.
Garnets are also useful in defining metamorphic facies of rocks. For instance, eclogite can be defined as a rock of basaltcomposition, but mainly consisting of garnet and omphacite.Pyrope-rich garnet is restricted to relatively high-pressure metamorphic rocks, such as those in the lower crust and in the Earth’s mantle. Peridotite may contain plagioclase, or aluminium-rich spinel, or pyrope-rich garnet, and the presence of each of the three minerals defines a pressure-temperature range in which the mineral could equilibrate with olivine plus pyroxene: the three are listed in order of increasing pressure for stability of the peridotite mineral assemblage. Hence, garnet peridotite must have been formed at great depth in the earth.Xenoliths of garnet peridotite have been carried up from depths of 100 km and greater bykimberlite, and garnets from such disaggegated xenoliths are used as a kimberliteindicator minerals in diamond prospecting. At depths of about 300 to 400 km and greater, a pyroxene component is dissolved in garnet, by the substitution of (Mg,Fe) plus Si for 2Al in the octahedral (Y) site in the garnet structure, creating unusually silica-rich garnets that have solid solution towards majorite. Such silica-rich garnets have been identified as inclusions within diamonds.
The largest documented garnet single crystal was an isometric block measuring ~2.3 m and weighing ~37.5 tons.[4] The news on larger garnet crystals found near Alice Springs, Northern Territory, Australia have not been confirmed.
Uses of garnets

Pendant in uvarovite, a rare bright-green garnet.
Pure crystals of garnet are used as gemstones. The gemstone varieties occur in shades of green, red, yellow and orange.[5]
In the USA it is known as the birthstone for January.[1] It is the state mineral of Connecticut.[6]It is also New York’s gemstone.[7]
Industrial uses
Garnet sand is a good abrasive, and a common replacement for silica sand in sand blasting. Mixed with very high pressure water, garnet is used to cut steel and other materials in water jets. Garnet sand is also used for water filtration media.
As an abrasive garnet can be broadly divided in two categories, blasting grade and water jet grade. The garnet, as it is mined and collected, is crushed to finer grains; all pieces which are larger than 60 mesh (250 micrometres) are normally used for sand blasting. The pieces between 60 mesh (250 micrometres) and 200 mesh (74 micrometres) are normally used for water jet cutting. The remaining garnet pieces that are finer than 200 mesh (74 micrometres) are used for glass polishing and lapping. Regardless of the application, the larger grain sizes are used for faster work and the smaller ones are used for finer finishes.
There are different kinds of abrasive garnets which can be divided based on their origin. The largest source of abrasive garnet today is garnet rich beach sand which is quite abundant on Indian and Australian coasts.[citation needed] This material is particularly popular due to its consistent supplies, huge quantities and clean material. The common problems with this material are the presence of ilmenite and chloride compounds. Since the material is being naturally crushed and ground on the beaches for past centuries, the material is normally available in fine sizes only. Most of the garnet at the Tuticorin beach is 80 mesh, and ranges from 56 mesh to 100 mesh size[citation needed].
River garnet is particularly abundant in Australia. The river sand garnet occurs as a placerdeposit.[citation needed].
Rock garnet is perhaps the garnet type used for the longest period of time. This type of garnet is produced in America, China and western India. These crystals are crushed in mills and then purified by wind blowing, magnetic separation, sieving and, if required, washing. Being freshly crushed, this garnet has the sharpest edges and therefore performs far better than other kinds of garnet. Both the river and the beach garnet suffer from the tumbling effect of hundreds of thousands of years which rounds off the edges.
Garnet has been mined in western Rajasthan for the past 200 years, but mainly for the gemstone grade stones. Abrasive garnet was mainly mined as a secondary product while mining for gem garnets and was used as lapping and polishing media for the glass industries. The host rock of the garnet here is garnetiferous mica schist and the total percentage of garnet is not more than 7% to 10%[citation needed], which makes the material extremely costly and non economical to extract for non-gemstone applications. |
|




























 Sodalite is named in reference to the sodium content it has. It is found in light to dark pure blue color and is well known in the semi-precious stone world. It is the only feldspathoid which contains chlorine.
Sodalite is named in reference to the sodium content it has. It is found in light to dark pure blue color and is well known in the semi-precious stone world. It is the only feldspathoid which contains chlorine.